RNA Clean-Up and Concentration Kits
For rapid and efficient clean-up and concentration of total RNA, including microRNA, without phenol

For research use only and NOT intended for in vitro diagnostics.

Version Available Here.
RNA Clean-Up and Concentration Kits
For rapid and efficient clean-up and concentration of total RNA, including microRNA, without phenol
Register today to receive an exclusive 15% off* on your first order.
Features and Benefits
- Clean-up & concentrate total RNA (including miRNA) in minutes
- Clean-up & concentrate RNA from TRIzol®, TRI Reagent®, etc
- Clean-up RNA from contaminants including enzymes, primers, nucleotides
- Rapid spin-column protocol, elute in 20 µL
- 96-well format for high throughput processing & Micro-Elute spin column formats for 8 µL are available
- Purified RNA is suitable for a variety of downstream applications, including Small RNA Sequencing. Find out more information on Norgen's NGS services
- Purification is based on spin column chromatography that uses Norgen’s proprietary resin separation matrix
These kits are designed to clean-up and concentrate RNA (including miRNA) from TRIzol® and TRI Reagent®, enzymatic reactions, in vitro transcription, labelling reactions, etc. These are robust kits for all clean-up and concentration purposes for up to 35 µg of RNA in solution. The purified RNA is of the highest purity and integrity, and can be used in a number of downstream applications. These kits purify all sizes of RNA, from large mRNA and ribosomal RNA down to miRNA, siRNA and lncRNA.
RNA Clean-Up and Concentration Kit (Spin Column)
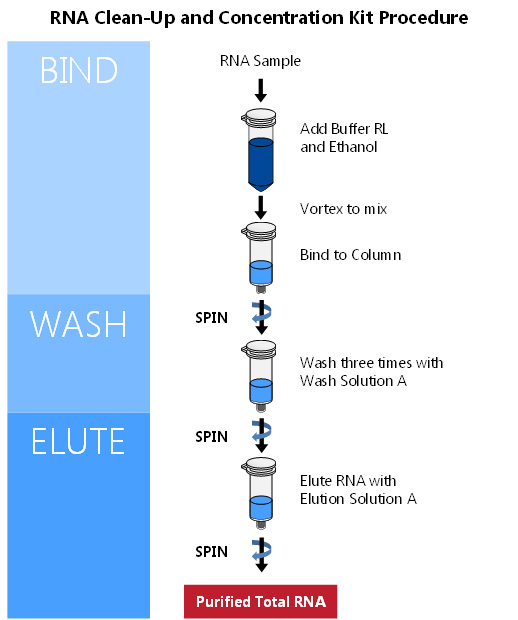
Norgen’s RNA Clean-Up and Concentration Kit provides a rapid method for the purification, cleanup and concentration of up to 35 μg of RNA isolated using different methods including phenol/guanidine-based protocols, and from various upstream enzymatic reactions such as DNase treatment and labeling. The kit elutes RNA in 20 µL. Complete 10 purifications in 20 minutes.
RNA Clean-Up and Concentration 96-Well Kit (High Throughput)
Norgen’s RNA Clean-Up and Concentration 96-Well Kit provides a rapid method for the purification, cleanup and concentration of up to 50 μg of RNA isolated using different methods including phenol/guanidine-based protocols, and from various upstream enzymatic reactions such as DNase treatment and labeling. The kit elutes RNA in 75 µL. Complete 10 purifications in 30 minutes.
RNA Clean-Up and Concentration Micro-Elute Kit (Micro-Elute)
Norgen’s RNA Clean-Up and Concentration Micro-Elution Kit provides a rapid method for the purification, cleanup and concentration of up to 10 μg of RNA isolated using different methods including phenol/guanidine-based protocols, and from various upstream enzymatic reactions such as DNase treatment, labeling and in vitro transcription. This kit is made for eluting RNA in smaller volumes of 8 µL for all types of downstream applications, for the highest concentration of RNA sample. Complete 10 purifications in 20 minutes.
Protocol
Details
Supporting Data
Figure 1. Effective Clean-Up to Produce High Quality Total RNA with Complete Size Diversity. Norgen's RNA Clean-Up and Concentration Kit effectively cleans up RNA isolated from phenol-based extractions without the loss of RNA diversity by retaining all RNA species including small RNAs. Total RNA was isolated from 5 x 108 E. coli using a competitor's phenol-based RNA extraction reagent. The resulting RNA was then purified using Norgen’s RNA Clean-Up and Concentration Kit. As controls, total RNA was extracted using both Norgen's Total RNA Purification Kit (#17200, no phenol required) and the phenol-based RNA extraction reagent only without any clean-up. Resolution of 7 µL of the 50 µL purified RNA on a 1X MOPS, 1.5% formaldehyde-agarose gel showed the RNA extracted with phenol was successfully cleaned-up by Norgen's RNA Clean-Up and Concentration Kit without the loss of small RNA species.
Figure 2. Effective Clean-Up to Produce High Quality Total RNA Compatible to Bioanalyzer Analysis. Total RNA was isolated from 0.75 million HeLa cells using a competitor's phenol-based RNA extraction reagent. The resulting RNA was then purified using Norgen's RNA Clean-Up and Concentration Kit. As controls, total RNA was extracted using both Norgen's Total RNA Purification Kit (#17200, no phenol required) or the phenol-based RNA extraction reagent only without clean-up. Resolution of 1 µL of the 50 µL purified RNA on an Agilent RNA Nano 6000 Chip showed that without clean-up, the RNA sample isolated using the phenol-based RNA extraction reagent only resolved poorly on a bioanalyzer. In contrast, RNA extracted with the phenol-based RNA extraction reagent followed by clean-up by Norgen's RNA Clean-Up and Concentration Kit resolved properly on the bioanalyzer showing excellent quality.
Figure 3. Effective Concentration and Detection of Low Amounts of RNA Input. Norgen's RNA Clean-Up and Concentration Kit can effectively concentration RNA inputs in the picogram range. Increasing amounts of HeLa RNA in 50 µL input volumes were concentrated to 20 µL using Norgen's RNA Clean-Up and Concentration Kit. A 10 µL aliquot of the purified RNA was then used as the template in a qRT-PCR reaction to detect the S14 gene. The amounts indicated on the graph correspond to the amount of RNA that was used as the input for the qRT-PCR reaction, demonstrating the consistent performance of the kit even in the picogram range.
Figure 4. Effective Clean-Up to Produce High Quality RNA Transcripts. Norgen's RNA Clean-Up and Concentration Kit effectively cleans up RNA transcripts with high recovery. Three RNA transcripts (1-3) of different sizes (266n, 161n and 419n, respectively) were produced using Norgen's RNA Clean-Up and Concentration Kit. The resulting RNA transcripts were then purified using Norgen's RNA Clean-Up and Concentration Kit. Resolution of 7.5 µL of the 50 µL cleaned RNA (C) as well as an equal volume of the uncleaned input (I) on a 1X MOPS, 1.5% formaldehyde-agarose gel showed the RNA transcript was successfully cleaned-up by Norgen's RNA Clean-Up and Concentration Kit.
Figure 5. Effective Clean-Up to Produce High Quality RNA Transcripts Compatible to Bioanalyzer Analysis. Norgen's RNA Clean-Up and Concentration Kit effectively cleans up RNA transcripts with high recovery. Three RNA transcripts (1-3) of different sizes (266n, 161n and 419n, respectively) were produced using Norgen's RNA Clean-Up and Concentration Kit. Resolution of 1 µL of the 50 µL cleaned RNA transcripts on an Agilent RNA Nano 6000 Chip showed that RNA transcripts cleaned by Norgen's RNA Clean-Up and Concentration Kit resolved properly on the bioanalyzer showing excellent quality.
Figure 6. Excellent Quality of Concentrated RNA. Total RNA isolated from HeLa cells (2 µg) was concentrated to 8 µL using the RNA Clean-Up and Concentration Micro-Elute Kit. The excellent quality is indicated by the electropherogram generated using the Agilent 2100 Bioanalyzer (RIN > 9). The concentrated RNA is a true 'total RNA' as can be observed by the presence of small RNA species.
Figure 7. High Recovery of Concentrated RNA. Total RNA isolated from HeLa cells (17.5 µL) was concentrated to 8 µL using the RNA Clean-Up and Concentration Micro-Elute Kit resulting in a 2-2.2 fold increase in RNA concentration, as was measured by Agilent 2100 Bioanalyzer quantification.
Figure 8. Concentration of Total RNA. The indicated amounts of HeLa RNA were concentrated using the RNA Clean-Up and Concentration Micro-Elute Kit. For each input amount, a 17.5 µL volume was processed and the RNA eluted in 8 µL, resulting in a 2.2 fold concentration. Three microliters of each eluate were used in 10 µL RT reactions with the oligo-dT primer, followed by real-time PCR using 3 µL of the resulting cDNA and primers specific for the human RPS15 gene. On average, amplifications of the concentrated RNA reached threshold 1 Ct value before the input RNA samples, which was expected based on the concentration factor. The lowest input amount used (240 pg) was only amplified when concentrated (most right sample in figure).
Figure 9. Concentration of miRNA. The indicated amounts of HeLa RNA were concentrated using the RNA Clean-Up and Concentration Micro-Elute Kit. Three microliters of each 8 µL eluate were used in 10 µL RT reactions with the miR-21-SLR primer, followed by real-time PCR using 3 µL of the resulting cDNA and the forward primer specific for miR-21.
Figure 10. Concentration of RNA prior to Next Generation Sequencing (NGS) applications. Total RNA was purified from 200 µL of plasma collected on EDTA blood tubes using Norgen's Total RNA Purification Kit (Cat. 17200) and eluted in 50 µL of elution solution. The same RNA was also concentrated two-fold using the Micro-Elute RNA Column by eluting in 25 µL of elution solution. Five microliters of both the RNA without additional concentration and the 2X concentrated RNA were used as inputs to generate RNA libraries (using the NEBNext® Small RNA Library Prep Set for Illumina® and following manufacturer’s instructions) for small RNA NGS on the MiSeq (Illumina) platform. A) The prepared small RNA libraries were visualized on a 6% TBE polyacrylamide gel, where the library prepared with 2X concentrated RNA contained more ligated/indexed miRNA cDNA (147-160 bp) products than the library prepared using the RNA without concentration. B) The cDNA was extracted from excised gel bands and interrogated using the Agilent 2100 Bioanalyzer (High Sensitivity DNA Assay). As would be expected based on input, the small RNA library prepared with the 2X concentrated RNA sample was approximately two times more concentrated than the library prepared with RNA without prior concentration (39.8 vs 21.2 nM, respectively).
|
Kit Specifications (Spin Column)
|
|
| Maximum Column Binding Capacity | 35 μg |
| Size of RNA Purified | All sizes, including small RNA (<200 nt) |
| Maximum Amount of Starting Material | 35 μg of RNA |
| Minimum Elution Volume | 20 μL |
| Time to Complete 10 Purifications | 20 minutes |
| Average Recovery | ≥ 90% |
Storage Conditions and Product Stability
All solutions should be kept tightly sealed and stored at room temperature. This kit is stable for 2 years after the date of shipment.
| Component | Cat. 23600 (50 preps) | Cat. 43200 (100 preps) | Cat. 25100 (192 preps) | Cat. 61000 (50 preps) |
|---|---|---|---|---|
| Buffer RL | 40 mL | 2 x 40 mL | 2 x 40 mL | 40 mL |
| Wash Solution A | 38 mL | 2 x 38 mL | 2 x 38 mL | 38 mL |
| Elution Solution A | 6 mL | 2 x 6 mL | 2 x 20 mL | 6 mL |
| Column Activation Solution | - | - | - | 30 mL |
| Micro Spin Columns | 50 | 100 | - | - |
| Micro-Elute RNA Spin Columns | - | - | - | 50 |
| 96-Well Plate | - | - | 2 | - |
| Adhesive Tape | - | - | 4 | - |
| Collection Tubes | 50 | 100 | - | 50 |
| 96-Well Collection Plate | - | - | 2 | - |
| Elution Tubes (1.7 mL) | 50 | 100 | - | 50 |
| 96-Well Elution Plate | - | - | 2 | - |
| Product Insert | 1 | 1 | 1 | 1 |
Documentation
(43200) RNA Clean-Up and Concentration Kit (Spin Column)- Protocol (100 prep)
(25100) RNA Clean-Up and Concentration 96-Well Kit (High Throughput) - Protocol (96-well)
(61000) RNA Clean-Up and Concentration Micro-Elute Kit (Micro-Elute) - Protocol (50 prep)
FAQs
Spin Column, High Throughput, Micro-Elute
Poor DNA recovery could be due to one or a combination of the following factors:
- Column has become clogged.
Do not exceed the recommended amounts of starting materials. The amount of starting material may need to be decreased if the column shows clogging below the recommended levels. See FAQ related to “Clogged Columns/wells” below.
- An alternative elution solution was used.
It is recommended that the Elution Solution A supplied with this kit be used for maximum RNA recovery.
- Ethanol was not added to the lysate.
Ensure that the appropriate amount of ethanol is added to the lysate before binding to the column.
- Ethanol was not added to the Wash Solution A.
Ensure that 90 mL of 96 - 100% ethanol is added to the supplied Wash Solution A prior to use.
Column/well clogging can result from one or a combination of the following factors:
- High amounts of RNA as input.
Ensure that no more than 50 µg of RNA are used as input.
- High amounts of genomic DNA present in sample.
The lysate may be passed through a 25 gauge needle attached to a syringe 5-10 times in order to shear the genomic DNA prior to loading onto the column.
- Centrifuge temperature too low.
Ensure that the centrifuge remains at room temperature throughout the procedure. Temperatures below 15°C may cause precipitates to form that can cause the columns to clog.
- Improper mixing of lysate and ethanol.
Ensure thorough mixing of the lysate with ethanol to prevent clogging and ensure efficient binding of RNA to the column.
- Presence of impurities in the sample.
Impurities such as cell debris or proteins can cause clogging. Ensure the sample is properly lysed and cleared before loading onto the column.
Degraded RNA may be caused by the following factors:
- RNase contamination.
RNases may be introduced during the use of the kit. Ensure proper procedures are followed when working with RNA. Please refer to “Working with RNA” at the beginning of this user guide.
- Procedure not performed quickly enough.
In order to maintain the integrity of the RNA, it is important that the procedure be performed quickly.
- Improper storage of the purified RNA.
For short term storage RNA samples may be stored at –20°C for a few days. It is recommended that samples be stored at –70°C for longer term storage.
If the RNA does not perform well in downstream applications, it may be due to one or more of the following:
- RNA was not washed three times with the provided Wash Solution A.
Traces of salt from the binding step may remain in the sample if the column is not washed three times with the Wash Solution A. Salt may interfere with downstream applications, and thus must be washed from the column.
- There is ethanol carryover.
Ensure that the dry spin under the Column Wash procedure is performed, in order to remove traces of ethanol prior to elution. Ethanol is known to interfere with many downstream applications.
The contamination with DNA or genomic DNA may be due to the use of large amounts of starting material. To address this issue, it is recommended to perform RNAse-free DNase I digestion as detailed in Appendix A. This step helps ensure the removal of any residual DNA, maintaining the purity of the RNA sample.
Citations
| Title | Bacillus pumilis Reveals a Remarkable High Resistance to Hydrogen Peroxide Provoked Oxidative Stress |
| Citation | PLoS One 2023. |
| Authors | T Handtke, R Schroeter, B Jurgen, K Methling, R Schluter, D Albrecht, S van Hijum, J Bongaerts, K Maurer, M Lalk, T Scheder, M Hecker, B Voigt |
| Title | Bacterial outer membrane vesicles induce a transcriptional shift in arabidopsis towards immune system activation leading to suppression of pathogen growth in planta |
| Citation | bioRxiv|Journal of Extracellular Vesicles 2023. |
| Authors | Laura Chalupowicz, Gideon Mordukhovich, Nofar Assoline, Leron Katsir, Noa Sela and Ofir Bahar |
| Title | Blocking of targetted microRNAs from next-generation sequencing libraries |
| Citation | Nucleic Acids Research Advance 2023. |
| Authors | BS Roberts, AH Hardigan, MK Kirby, MB Fitz-Gerald, CM Wilcox, RP Kimberly, RM Myers |
| Title | Cardiac effects of myoregulin in ischemia-reperfusion |
| Citation | Peptides 2023. |
| Authors | Sarah Appleby a 1, Hamish M. Aitken-Buck b 1, Mark S. Holdaway a, Mathew S. Byers a, Chris M. Frampton a, Louise N. Paton a, A. Mark Richards a c d, Regis R. Lamberts b, Christopher J. Pemberton a |
| Title | Characterization of the global impact of low temperature gas plasma on vegetative microorganisms |
| Citation | Proteomics 2023. |
| Authors | Theresa Winter1, Jörn Winter2, Martin Polak2, Kathrin Kusch1, Ulrike Mäder1,3, Rabea Sietmann1, Jörg Ehlbeck2, Sacha van Hijum3*, Klaus-Dieter Weltmann2, Michael Hecker1, Harald Kusch1# |
| Title | Circulating Micrornas Predict Survival of Patients with Tumors of Glial Origin |
| Citation | EbioMedicine 2023. |
| Authors | Drusco, A., Fadda, P., Nigita, G., Fassan, M., Bottoni, A., Gardiman, M. P., ... & Casini, B. (2018). |
| Title | Circulating miRNAs in Murine Experimental Endometriosis Decreased Abundance of let-7a |
| Citation | Reproductive Sciences 2023. |
| Authors | Seifer, B. J., Su, D., & Taylor, H. S |
| Title | Common versus noble- Bacillus subtilis differentially responds to air and argon gas plasma |
| Citation | Proteomics 2023. |
| Authors | T Winter, J Bernhardt, J Winter, U Mader, R Schluter, KD Weltmann, M Hecker, H Kusch |
| Title | Effect of adenovirus infection on transgene expression under the adenoviral MLP/TPL and the CMVie promoter/enhancer in CHO cells |
| Citation | Journal of Genetic Engineering & Biotechnology 2023. |
| Authors | El-Mogy, M. A., Abdalla, M. A., Misic, V., & Haj-Ahmad, Y. (2017) |
| Title | Expression of microRNAs and their target genes in melanomas originating from gynecologic sites |
| Citation | plosone 2023. |
| Authors | Mallory J. DiVincenzo ,Colin D. Angell ,Lorena P. Suarez-Kelly,Casey Ren,Zoe Barricklow,Maribelle Moufawad,Paolo Fadda,Lianbo Yu,Floor J. Backes,Kari Ring,Anne Mills,Craig Slingluff,Catherine Chung,Alejandro A. Gru,William E. Carson III |