No More Monkey Business
Since its discovery in the 1980s, HIV (Human Immunodeficiency Virus) has had a significant impact on countless lives across the world. As of today, more than 37 million people worldwide are infected according to statistics generated by the World Health Organization. This is roughly the entire population of Canada, although to date there has been no effective cure and many continue to suffer from their affliction. For the past 35 years, all of these patients have been hearing the same thing from their physicians: wait. Wait for a new development, a breakthrough or innovative research to be undertaken. Research on HIV vaccine development has been limited to using non-human primates (NHPs) and their equivalent of HIV: SIV (Simian Immunodeficiency Virus). This contributes to the lack of progression in the field, but now patients should have reason to be optimistic that the elusive HIV vaccine might be accessible by 2021.
Published in Science Translational Medicine, a recent paper written by Ehrenberg et. al out of the US Military HIV research program aims to bridge the gap between preclinical and clinical data surrounding the HIV vaccine hype. They are pushing to advance research by delineating a new approach for evaluating future vaccine candidates as well as aid in the empirical design of a truly efficacious one. Two prior studies on rhesus monkeys and an SIV vaccine has shown a protective effect from the virus, although not all of the monkeys tested showed the same response. As well, the only human clinical trial of an HIV vaccine at the time had similar findings–an only partially efficacious treatment. Erhrenberg and his team embarked on a journey to figure out why not all of the subjects showed a similar vaccine evoked immune response.
Peripheral blood mononuclear cells (PBMCs) available from two prior studies on rhesus monkeys as well as one from humans were analyzed. Total RNA was isolated from these PBMCs by using the Single Cell RNA Purification Kit (Norgen Biotek Corp.) and then sequenced via Next Generation Sequencing (NGS).
What was found is that the antibody producing B cells from those with protection from the virus all showed a similar gene expression signature after performing gene set enrichment analysis. Figure 1 below elucidates this key finding.
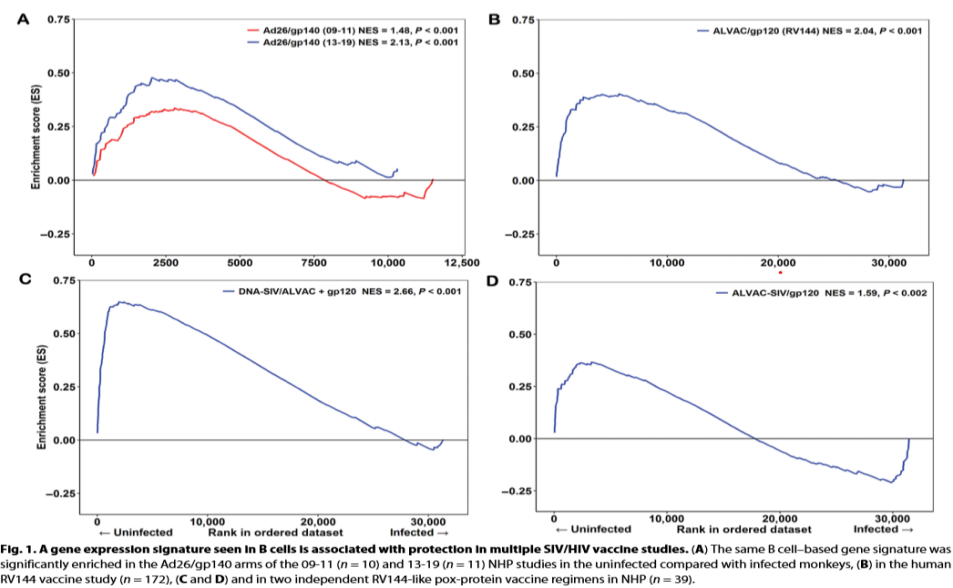
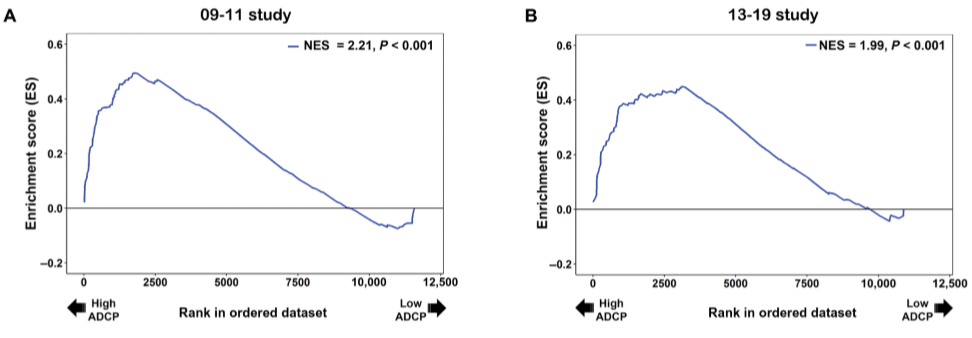
Now that it is known subjects who are protected from the virus after the vaccine show similar gene expression, it begs the question of whether or not acquisition can be predicted. Of the 58 enriched genes in the first NHP study, 53 passed the gene expression filtering step in the second NHP study. A prediction model based off these genes was then able to both discriminate infected from uninfected animals as well as predict protection from one study to the other. This is valuable information, although what needs to be proved is that this increased gene expression is leading to an increased magnitude of cellular and antibody immune response in treatment groups. Immune correlates such as antibody-dependent cellular phagocytosis (ADCP), Fc antibody polyfunctionality, and immunoglobulin G breadth were measured, although ADCP was the only immune response associated with the B cell signature in both NHP studies. Panels A and B below depict this clearly.
NORBLOG
Want to hear more from Norgen?
Join over 10,000 scientists, bioinformaticians, and researchers who receive our exclusive deals, industry updates, and more, directly to their inbox.
For a limited time, subscribe and SAVE 10% on your next purchase!
SIGN UP
A network analysis to identify relationships between the 53 enriched genes in the B cell signature and their known role and function was then performed. Two genes of interest were identified that were associated with reduced risk on infection: OGFRL1 and TNFSF13. The latter gene belongs to the tumour necrosis factor ligand superfamily, which modulates HIV replication.
Prospective research into this transcriptional signature would be valuable for future human clinical trials and various vaccine regimens. Often times looking at a single gene to start with ends up without a solid conclusion and in no ways expedites treatment options. Using large scale transcriptomic approaches will be helpful to the end goal: virus eradication.
The upcoming year will be pertinent for the development of an HIV vaccine. There are presently three HVTN (HIV Vaccine Trials Network) clinical trials underway: HVTN 702–evaluating the preventive vaccine efficacy, safety and tolerability based out of South Africa, HVTN 705–a multicenter study across sub-Saharan Africa and HVTN 706–testing efficacy of a hererologous vaccine regimen across the world from the US to South America and also Europe. These clinical trials would benefit immensely from looking at gene signatures, such as was conducted in this study.
With more well conducted research, the need for future studies might one day become unnecessary. It may be true that good things come to those who wait, but great things come to those who don’t. HIV Patients across the world should find comfort in the fact that researchers are not waiting any longer.
-
Ehrenberg, P. K., Shangguan, S., Issac, B., Alter, G., Geretz, A., Izumi, T., … & Creegan, M. (2019). A vaccine-induced gene expression signature correlates with protection against SIV and HIV in multiple trials. Science translational medicine, 11(507), eaaw4236.