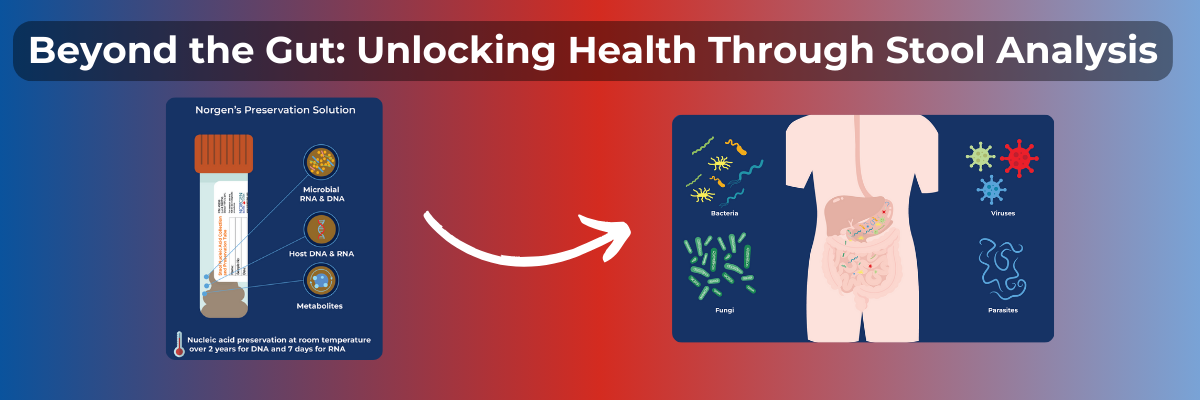
In the past, diagnoses were limited to what could be observed by an attending physician with their own senses. As one might expect, this resulted in wildly varying levels of patient care and unconventional tests and treatments, many of which unfortunately involved livestock. Fortunately, the modern era has seen vast advancements in the area of molecular biology, and by extension, medical diagnosis. Today we have definitive tests for many conditions and diseases ranging from infertility to cancer. Recently, the world has seen a dire need for a rapid means of determining if a subject is positive for COVID-19. The current gold standard methods for diagnosing COVID-19 are antigen testing and polymerase chain reaction (PCR).
Antigen Testing
 The most common antigen test is likely quite familiar to just about everyone as it resembles a pregnancy test. COVID-19 antigen tests function in the same way and rely on the specific binding of an antigen (a protein displayed on the surface of the virus) in the sample to antibodies (yet more proteins, usually animal-sourced) immobilized in the spots where the indicator lines appear.1 To facilitate detection, secondary antibodies also bind to the antigen and either fluoresce or change color in response.1 The level of colour change or fluorescence can also be used to quantify the level of antigen present in the sample. In the case of the COVID-19 antigen test, the sample comes from the patient’s upper respiratory tract, usually through an oropharyngeal or nasopharyngeal swab, as pictured to the right.
The most common antigen test is likely quite familiar to just about everyone as it resembles a pregnancy test. COVID-19 antigen tests function in the same way and rely on the specific binding of an antigen (a protein displayed on the surface of the virus) in the sample to antibodies (yet more proteins, usually animal-sourced) immobilized in the spots where the indicator lines appear.1 To facilitate detection, secondary antibodies also bind to the antigen and either fluoresce or change color in response.1 The level of colour change or fluorescence can also be used to quantify the level of antigen present in the sample. In the case of the COVID-19 antigen test, the sample comes from the patient’s upper respiratory tract, usually through an oropharyngeal or nasopharyngeal swab, as pictured to the right.
Want to hear more from Norgen?
Join over 10,000 scientists, bioinformaticians, and researchers who receive our exclusive deals, industry updates, and more, directly to their inbox.
For a limited time, subscribe and SAVE 10% on your next purchase!
SIGN UP
PCR Testing
 PCR testing makes millions of copies of a specific sequence or sequences in the genetic material present in the sample allowing for detection of a much lower initial viral load. These copied sequences can then be used to diagnose a patient, either by their presence (positive) or absence (negative). In the case of COVID-19, the genetic material is comprised of Ribonucleic Acid (RNA) which is relatively fragile, as opposed to Deoxyribonucleic Acid (DNA), which is far more stable.3 In order to perform the test, which can only be performed on DNA, a conversion from the former to the latter must be performed.3 This is done through the use of an enzyme called reverse transcriptase, which makes a copy of the RNA out of DNA. Once the complementary DNA, or cDNA, has been obtained, the PCR can proceed. The main components consist of the Taq DNA polymerase, the template, the primers, and loose nucleotides in the form of deoxynucleoside triphosphates (dNTPs).3 The polymerase can best be visualized as a piece of machinery whose job is to assemble new segments of DNA. Like a piece of machinery, the polymerase requires instructions such as where to start and the sequence of the new strand. The template is the piece of cDNA created from the sample and provides the sequence of the new strand. Everyone knows the familiar double helix profile of DNA, which gives a good indication of its structure. DNA is composed of two complementary strands of linked nucleotides in matched pairs of A/T and C/G. Thus if one strand is present, the polymerase can produce the opposite strand. Primers give the start points and are short segments of DNA with the opposing sequence of base pairs on the template strand at the beginning of the segment of interest. Finally, dNTPs can be considered the fuel. They are single nucleotides which the polymerase links together into the final product. The actual process of the PCR involves heating and cooling the mixture in order to sequentially separate the complementary strands, adhere the primers, and finally allow the polymerase to replicate the segment of interest.3 By repeating this process, the original strand is exponentially amplified.3
PCR testing makes millions of copies of a specific sequence or sequences in the genetic material present in the sample allowing for detection of a much lower initial viral load. These copied sequences can then be used to diagnose a patient, either by their presence (positive) or absence (negative). In the case of COVID-19, the genetic material is comprised of Ribonucleic Acid (RNA) which is relatively fragile, as opposed to Deoxyribonucleic Acid (DNA), which is far more stable.3 In order to perform the test, which can only be performed on DNA, a conversion from the former to the latter must be performed.3 This is done through the use of an enzyme called reverse transcriptase, which makes a copy of the RNA out of DNA. Once the complementary DNA, or cDNA, has been obtained, the PCR can proceed. The main components consist of the Taq DNA polymerase, the template, the primers, and loose nucleotides in the form of deoxynucleoside triphosphates (dNTPs).3 The polymerase can best be visualized as a piece of machinery whose job is to assemble new segments of DNA. Like a piece of machinery, the polymerase requires instructions such as where to start and the sequence of the new strand. The template is the piece of cDNA created from the sample and provides the sequence of the new strand. Everyone knows the familiar double helix profile of DNA, which gives a good indication of its structure. DNA is composed of two complementary strands of linked nucleotides in matched pairs of A/T and C/G. Thus if one strand is present, the polymerase can produce the opposite strand. Primers give the start points and are short segments of DNA with the opposing sequence of base pairs on the template strand at the beginning of the segment of interest. Finally, dNTPs can be considered the fuel. They are single nucleotides which the polymerase links together into the final product. The actual process of the PCR involves heating and cooling the mixture in order to sequentially separate the complementary strands, adhere the primers, and finally allow the polymerase to replicate the segment of interest.3 By repeating this process, the original strand is exponentially amplified.3
These cycles were originally performed manually, moving the samples from one water bath to another, though fortunately this process has been automated.2 On average, PCR tests undergo 40 of these loops, creating over one trillion copies of the initial strand.3 As a sense of scale, one trillion seconds is equivalent to 30,000 years, or all the way back to the Upper Stone Age. This massive amount of amplification allows for detection of the virus at extremely low concentrations, resulting in both a reduced requirement for sample size and a high degree of accuracy in detection. This detection is accomplished through the use of fluorescent markers attached to a probe in a process known as quantitative PCR, or qPCR. This probe is yet another short segment of complementary DNA that adheres to the template and is destroyed when the copy is made. When it is destroyed, the marker fluoresces. When the level of fluorescence reaches the point that it is detectable, the cycle threshold (Ct) has been reached and can be used to calculate the concentration of the original template in the sample.
Why Choose PCR Over Antigen Testing?
Theoretically, only one copy of the DNA being studied is required for detection, as it is amplified millions of times. Antigen testing requires thousands of virions to be present in the sample to have a positive result.1 This is detrimental for early detection, especially in asymptomatic cases as oftentimes the viral load in the sample will be too low to properly register, leading to a false negative. Conversely, false-positive tests are also more likely with antigen-based tests.4 This is due to the fact that related viral species may bind in the place of the SARS-CoV-2 virus, thereby eliciting the colour or fluorescence change.4 A false positive test result can be a traumatic experience, possibly resulting in financial, psychological and even physical harm.4 Every possible effort should be made to ensure that all positive test results are accurate to avoid unnecessary anguish.
Parting Thoughts
Current Ontario policy states that any positive rapid antigen test should be confirmed through a subsequent PCR test. Though it may take a little longer to get your results, the increased security of the higher sensitivity provides peace of mind not given with the antigen test. Norgen’s saliva-based qPCR test provides affordable, quick, and accurate COVID-19 testing for both businesses and individuals. It merely requires the individual to spit into a tube, add the provided preservative and ship it to Norgen for processing and analysis by our experienced scientists. Within 24 to 48 hours, you will have your result, and with it, your peace of mind.