COVID19-Specific Biomarkers Found in Plasma cfDNA
Cell-free DNA (cfDNA) refers to all circulating DNA present in the bloodstream which may originate from apoptotic cells as a part of the natural cell turnover, from cancer cells or fetal cells. A number of studies have highlighted the utility of cfDNA analysis for genetic profiling of various types of cancer, non-invasive prenatal testing (NIPT), and just recently, for monitoring organ-specific damage in systemic diseases like COVID19.
It is widely known that SARS-CoV-2, the causative agent of COVID19, primarily affects the human alveoli epithelial cells, but evidence of systemic disease with multi-organ involvement is emerging. Researchers at Cornell University have spearheaded this new avenue of cfDNA screening by developing a blood test to broadly quantify cell, tissue, and organ-specific injury due to COVID19, using genome-wide methylation profiling of circulating cell-free DNA in plasma (Cheng et al., 2020).
Cheng et al. (2020) found evidence for lung, liver, and kidney injury in hospitalized patients with COVID19, in line with the diverse clinical manifestations of the disease. Interestingly, the level of lung-specific cfDNA in plasma was similar to the levels observed in lung transplant patients that suffer acute lung transplant rejection and lung cancer patients. They also reported a striking correlation between the total abundance of circulating cfDNA in plasma and the WHO ordinal scale for disease progression. After comparing cfDNA signatures for COVID-19 patients as a function of disease severity with that of healthy individuals, they found that erythroblast (immature red blood cell) cfDNA proportions at any time point are predictive of in-hospital mortality (19.6% vs 4.1%, p value = 0.0004, Wilcoxon).
The fact that cfDNA can be quantified within a few hours of collection at a low cost, is truly groundbreaking as it can be used in the context of clinical trials and patient prognosis. Despite the utility of cfDNA as an efficient biomarker, there are many challenges that need to be overcome for cfDNA testing to be implicated in routine practice. Firstly, when cfDNA is being used for prenatal testing or cancer screening/monitoring, the cfDNA which needs to be analyzed constitutes a very small fraction of the total cfDNA present in the blood of the patient. This makes isolation and analysis of the desired fragment (~140-180 bp) very difficult as it is prone to be masked by any high molecular weight genomic DNA contamination. Secondly, there is no standardized protocol for cfDNA extraction and analysis. Differences between commercially available extraction kits can produce different yields because most have different sensitivities and binding affinities for the wide range of cfDNA species that exist.
Harnessing the full potential of cfDNA for these purposes thus requires significant technological refinement of the methods at the point-of-care (i.e. collection and preservation of samples) and at the lab bench (i.e. extraction of concentrated cfDNA).
NORBLOG
Want to hear more from Norgen?
Join over 10,000 scientists, bioinformaticians, and researchers who receive our exclusive deals, industry updates, and more, directly to their inbox.
For a limited time, subscribe and SAVE 10% on your next purchase!
SIGN UP
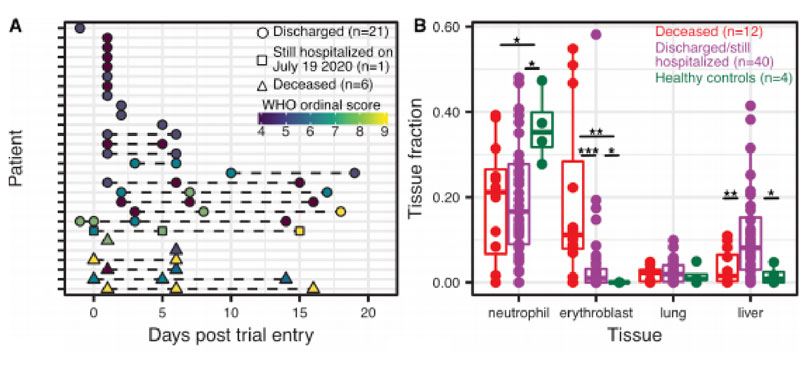
Figure 1. Randomized control trial cohort from McGill University Health Centre. A) Patient sample-collection map by day of enrollment into the study. B) The relative proportion of cfDNA derived from four cell and tissue types (neutrophil, erythroblast, lung, liver) by hospitalization status (p-values calculated using a Wilcoxon test; * :p-value < 0.05; ** :p-value < 0.01; *** :p-value < 0.001). Note the difference in erythroblast cfDNA profiles between healthy and non-healthy patients in the figure on the right.
Explore Norgen’s Solutions For Cell-Free DNA Collection, Stabilization, And Extraction
-
Cheng, A.P., Cheng, M.P., Gu, W., Lenz, J.S., Hsu, E., Schurr, E., Bourque, G., Bourgey, M., Ritz, J., Marty, F. and Chiu, C.Y.,2020. Cell-Free DNA in Blood Reveals Significant Cell, Tissue and Organ Specific injury and Predicts COVID-19 Severity.medRxiv.