What is IBS?
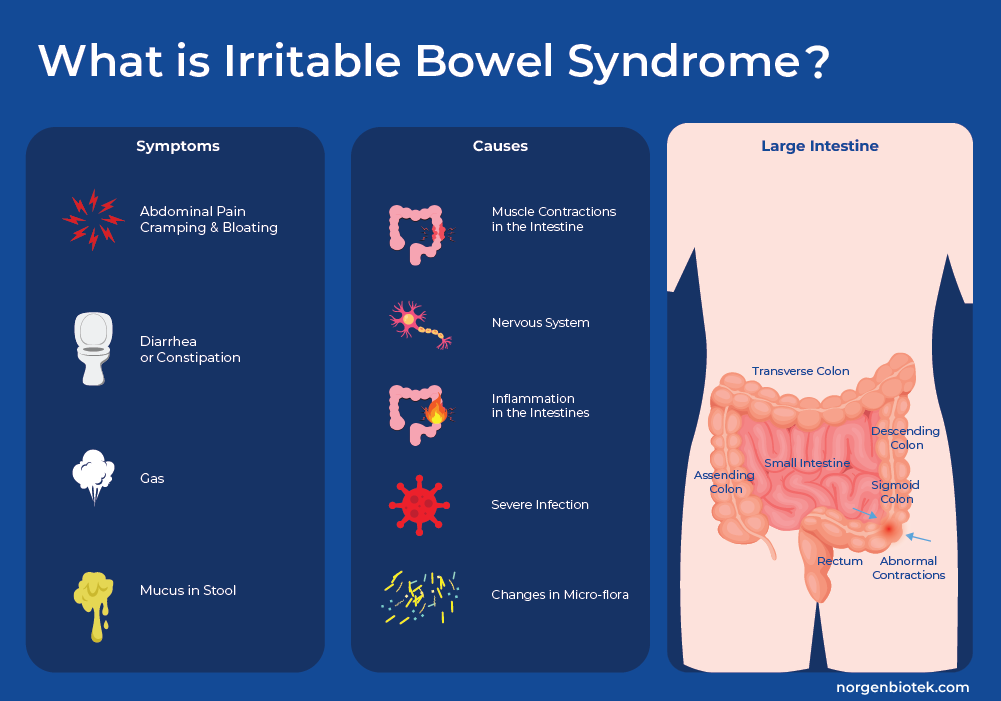
Irritable Bowel Syndrome (IBS) is a gastrointestinal disorder that is defined by problems with motility (how GI muscles move food through the digestive system), and sensitivity (how the brain interprets signals from the intestines). This leads to a spectrum of symptoms including abdominal pain, diarrhea, constipation, bloating, and mucus in the stool. These symptoms can persist for a while, but also come and go over time. IBS is frequently accompanied by anxiety and/or depression, and symptom onset or flare-ups is often associated with stressful life events. The link between these gastrointestinal symptoms, increased pain perception and mental health is so strong that many consider this a disorder of the gut-brain interaction.1 IBS can be classified into four different subtypes depending mainly on the predominant stool pattern: IBS with diarrhea (IBS-D), IBS with constipation (IBS-C), and IBS mixed (IBS-M) which experiences a combination of stool types.
Want to hear more from Norgen?
Join over 10,000 scientists, bioinformaticians, and researchers who receive our exclusive deals, industry updates, and more, directly to their inbox.
For a limited time, subscribe and SAVE 10% on your next purchase!
SIGN UP
It is hard to estimate the global prevalence of this disorder due to heterogeneity in reporting2, but in the United States different sources estimate that between around 6%3-16%4 of the adult population is affected. It is the most common reason for a visit to a gastrointestinal specialist. It is most often a chronic disease, with symptoms so severe that it can restrict individuals ability to carry out day-to-day tasks. It is estimated that individuals with IBS restrict their activities up to 73 days a year resulting in significant direct and indirect costs to society, from medical expenses to absenteeism. These costs have been estimated to total USD 21 Billion a year.
Molecular Mechanisms Underlying IBS
Many causative factors and triggers for IBS have been identified, including genetic susceptibility, increased mucosal permeability, and altered gut microbiome. Moreover several processes that result in visceral hypersensitivity and perturbations of gastrointestinal motor and sensory functions have been recognized and can lead to IBS symptoms. This blog will go into a more detailed examination of a few of these factors.
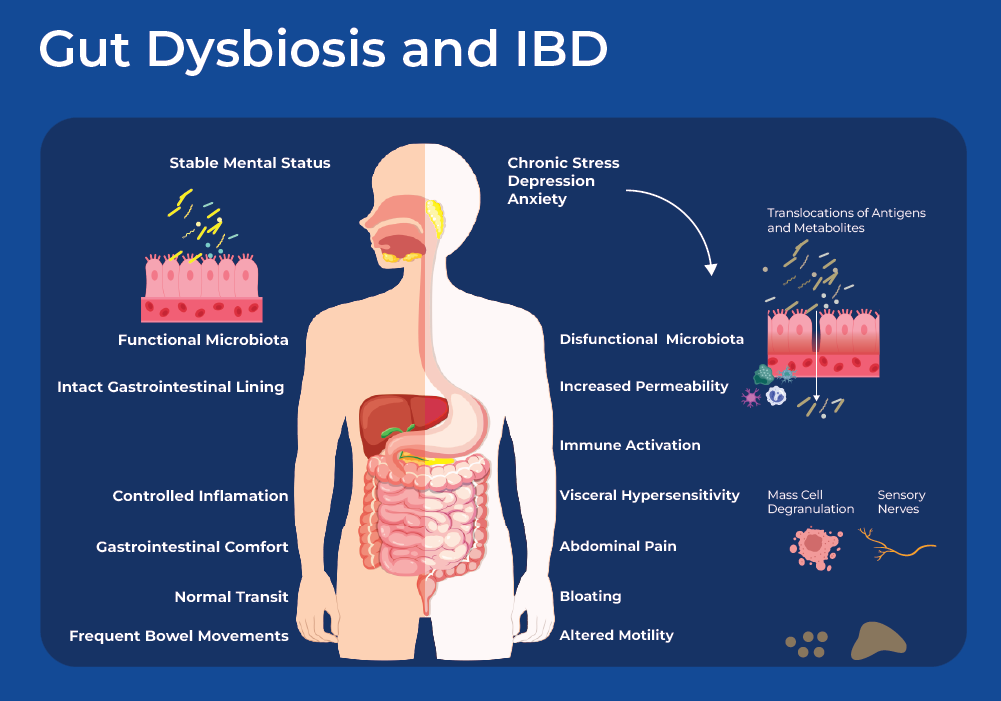
Gut Microbiome Dysbiosis
The human gut is home to a vast community of prokaryotes, fungi, viruses and protists. Through complex metabolic, neural, and immune interaction networks the microbiome is involved in metabolism of food, synthesis of vitamins, and regulating immune homeostasis. Many studies have shown that an imbalance in the microbiome can lead to IBS symptoms. For example, meta-analysis of large cohort and case-control studies suggests that antibiotics pose a risk factor for functional gastrointestinal disorders such as Irritable Bowel Syndrome.5 Moreover, infection with gastrointestinal virus or bacteria is a risk factor for the development of IBS.6, 7 Small intestinal bacterial overgrowth (SIBO) is also frequently observed in the small intestine of IBS patients. SIBO characterized by the overgrowth of colonic type bacteria, such as Klebsiella, Enterococcus, and Escherichia.8 In addition to these more drastic shifts in microbial communities, meta-analysis have found IBS patients to have decreased bacterial diversity and slightly different composition than that of healthy individuals. A meta-analysis of IBS studies, found lower levels of fecal Lactobacillus and Bifidobacterium, along with higher levels of Escherichia coli and Enterobacter in feces samples obtained from IBS patients.
The gut microbiome is directly connected to the nerve and immune systems, the enteric nervous system in the gut wall contains about 170 million nerve cells,10 and gut-associated lymphoid tissues (GALT) represent more than 70% of the total immune system.11 The microbes and metabolic pathways interact with the neuroendocrine, and immune system forming the gut-brain axis. Together the gut-brain axis regulates many GI physiological functions, including motility, secretion, immunity and thereby inflammatory processes. The interaction is two-way as microbes can directly interact with the host's cells, modulating specific immune, metabolic and neuronal pathways activating both immune and non-immune cells that can participate in IBS pathogenesis. In the other direction, inflammation-induced alterations in the intestinal environment disrupt the composition of its resident microorganisms, resulting in dysbiosis.
One interesting study demonstrated that IBS has a microbial pathogenesis by performing a fecal transplant from IBS patients to healthy germ-free mice who subsequently started exhibiting IBS symptoms. Their findings revealed that transplanting fecal microbiota from IBS-D patients with anxiety led to accelerated gastrointestinal transit (diarrhea), low-level inflammation, and anxiety-like behaviors. Mice receiving the IBS-D fecal microbiota showed a taxonomically similar microbial composition to that of mice receiving the healthy control fecal microbiota. However, IBS-D mice showed different serum metabolomic profiles.12
Gut-Brain-Axis
As mentioned there is a large neurological component to IBS with many patients having symptom onset after stressful life events, and subjects noting symptom increase after or during depression. There is much evidence supporting the influence of the microbiome through the gut-brain axis. One avenue by which the gut microbiome influences the brain is through the enteric nervous system, and in the other direction the composition and function of the microbiome can be affected by the central nervous system. Many studies have found correlations between bacterial taxa and microbiome-derived metabolites, enzymes, neurotransmitters and neuroendocrine factors on the pathogenesis of IBS.
Neurotransmitters such as serotonin (5-hydroxytryptamine), dopamine, γ-aminobutyric acid (GABA), and histamine, have significant involvement in Irritable Bowel Syndrome (IBS), particularly concerning visceral sensitivity and gastrointestinal motility. As mentioned, IBS is associated with gut microbiome dysbiosis, which can in turn disrupt the normal function of intestinal mast cells. These mast cells respond to inflammatory stimuli and release mediators that interact with nearby endocrine cells and nerve fibers, triggering the release of neurotransmitters which can enter the vagus nerve or the circulatory system. This affects intestinal motility and sensation, causing heightened sensitivity in visceral afferent nerves, the intestinal nerve, and the intestinal nervous system, leading to gastrointestinal dysfunction and symptoms characteristic of Irritable Bowel Syndrome (IBS).13
Gut Virome
Viruses are the most abundant organisms in the gut being about 10 times more abundant than bacteria. They are important members of the gut microbiome as bacteriophages interact with bacteria and eukaryotic-targeting viruses can interact with host cells as well as fungi. Phages directly affect community structure through targeted killing of host prokaryotes. Moreover phages can indirectly cause an immune response as phage-mediated bacterial lysis releases bacterial products.
A recent study using Norgen's phage DNA isolation kit (Cat.46800) combined with shotgun sequencing of virus like particles, showed correlations between specific viral groups and IBS as well as decreased viral diversity.14 Several studies have shown Caudovirales was increased in subjects with IBS, concomitant with decreased diversity and richness of bacterial species.15 More specific examples of phages targeting beneficial bacteria was noted in a study where IBS subjects had high prevalence of Feaclibacterium prausnitzii phages, and lower concentrations of F. prausnitzii bacteria.16 In addition to bacteriophages, eukaryotic viruses have also been associated with intestinal inflammation. Anelloviridae is more prevalent in adults with IBD as well as children with early-onset IBD.15,17 Some of these these findings were based on data derived from mucosal biopsies, highlighting the possibility of a direct interaction of the eukaryotic viruses with the host's cells.
Moreover, by integrating metagenomic sequencing of virus-like particles with host transcriptomics and metabolite analysis, researchers identified correlations between the virome and host gene expression. Many of these genes were related to immune response and infection, for example toll-like receptor 2 (TLR2), cluster of differentiation 4 (CD4). They found specific bacteriophage groups (Podoviridae and Siphovirdae) to be associated with host genes, including PERP and zinc finger FYVE-type containing (ZFYVE28), that play a role in epithelial barrier function.18
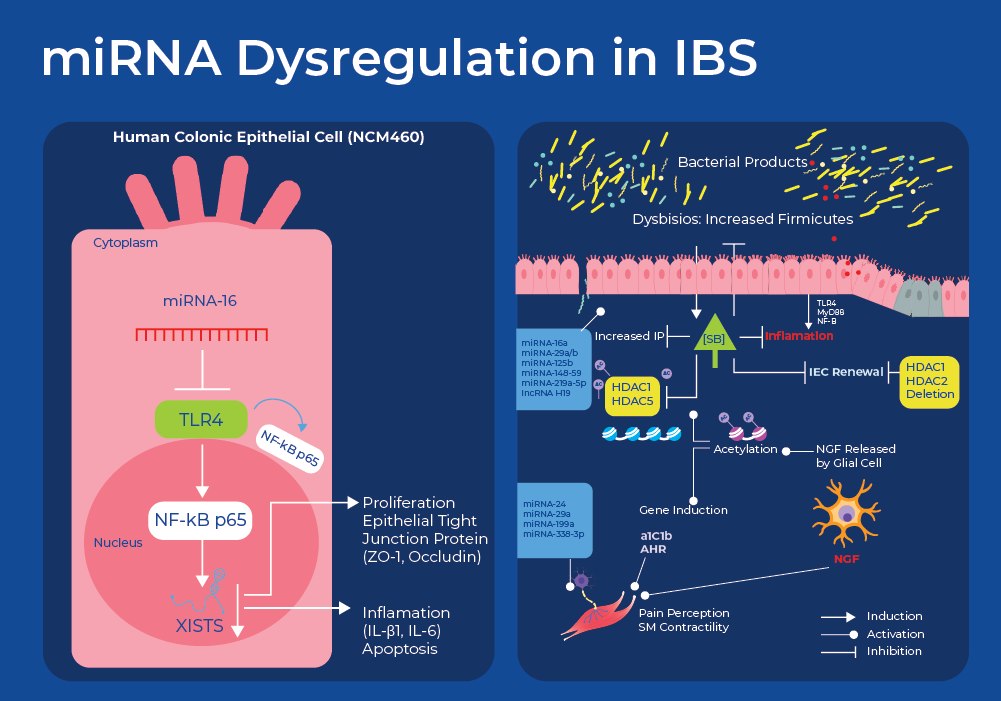
miRNA Dysregulation in IBS
IBS has traditionally been considered as a functional GI disorder defined by clinical manifestations lacking specific and sensitive biological markers. However, current research has identified differentially expressed mRNA and miRNAs that not only explain the pathology but could also be used as biomarkers for diagnosis and monitoring treatment efficacy. Research has shown differential gene expression in patients with IBS. A recent study identified 23 differentially expressed genes in IBD patients. Many of these genes were involved in serotonin regulation and some of these genes are regulated by vitamin D. This vitamin D finding is interesting and supported by other research that suggests that vitamin D deficiency is prevalent in individuals with IBS.19,20 Researchers are now also starting to identify some of the miRNAs involved in regulating this differential expression.
The first study implicating an miRNA in IBS involved miRNA-mediated regulation of a serotonin receptor. Mutation analysis on over 200 IBS patients and 100 healthy controls identified a mutation in the serotonin receptor encoding gene (5-HT) that resulted in hindered binding of a cognate miRNA (miR-510), thus decreasing repression and increasing the expression of the receptor. The variant showed a highly significant association with female IBS-D in two independent cohorts from the UK and Germany.21 Serotonin plays a pivotal role as both a neurotransmitter and a paracrine signaling molecule. It exerts direct and indirect influence on intestinal motor and secretory functions, potentially causing either constipation or diarrhea when abnormalities arise. Additionally, serotonin regulates the sensation and perception of visceral stimulation at both peripheral and central locations.22
Another miRNA pathway that has been implicated in IBS regulates tight junctions that are involved in controlling intestinal permeability. The miRNAs hsa-miR-125b-5p and hsa-miR-16 were found to regulate the expression of the tight junction genes CGN (cingulin) and CLDN2 (claudin-2), respectively. RNA-seq of intestinal tissue samples showed that the protein expression of CGN and CLDN2 showed an increase in individuals with IBS-D, while the levels of the respective targeting miRNAs decreased. Researchers also found correlations between the expression levels of the miRNAs with physiological factors such as bowel dysfunction, perceived stress, depression, and the number of mast cells exhibited.23
miRNA dysregulation was also shown to be linked to abdominal pain as decreased miR-199 expression was shown to increase visceral pain in patients with IBS through translational upregulation of transient receptor potential vanilloid type 1 (TRPV1).24 Increased neuronal expression of TRPV1 has been reported in colonic biopsies of patients with IBS. TRPV1 modulates visceral sensitivity and mechanosensation in animal models of IBS following stress or colonic inflammation.
Moreover miRNA-based regulation also has an effect on motility through regulation of Regulators of G protein signaling (RGS) proteins. RGS proteins regulate the magnitude and duration of signals initiated by several types of G protein-coupled receptor (GPCR) occurring in the GI tract, including opioid, cannabinoid and serotonin (5-HT) receptors. Analysis of exosomal RNA from IBS patients revealed elevated levels of miR-148b-5p and and subsequent cell culture experiments showed that increased miR-148b-5p decreased the levels of its predicted target RGS2 and in-turn, affected cell permeability.25
More recently, researchers used Norgen's patented SiC technology to extract and analyze RNA from the serum of IBS-D patients. They identified another miRNA pathway (miR-16) involved in IBS pathogenesis through the Toll-Like Receptor 4 (TLR4) signal pathway. TLR4 plays an important role in initiating the innate immune response and its activation by bacterial endotoxins is responsible for chronic and acute inflammatory disorders. Analysis of serum derived RNA showed that IBS-D patients had decreased levels of miR-16. Moreover, using a mouse model researchers showed decreased miR-16 along with increased cytokine levels. They also demonstrated that overexpression of miR-16 boosted intestinal epithelial cell viability, preserved tight junction integrity, and suppressed cell apoptosis and inflammation. This shows the role of miR-16 in mitigating mitigates IBS-D symptoms and suggests it as a potential therapeutic target for managing IBS-D.26
Treatment for IBS
Irritable Bowel Syndrome is a heterogeneous disorder with a gradient of symptoms based on irregular GI movement and pain. Several factors have been implicated in the pathophysiology of IBS, including the gut-brain axis, microbiome dysbiosis, genetic factors and visceral hypersensitivity. Moreover, it is likely that even among patients with the same symptoms, the underlying pathophysiology may differ. This means that there is no standard treatment for IBS.1 Treatment guidelines use a combination of therapies based on a patient's symptoms and their response to certain treatments. These treatments can include: dietary changes, probiotics, antispasmodics, antidiarrheals,laxatives, fiber supplements and psychotherapy. This blog will touch on the physiological effects of psychotherapy treatment.
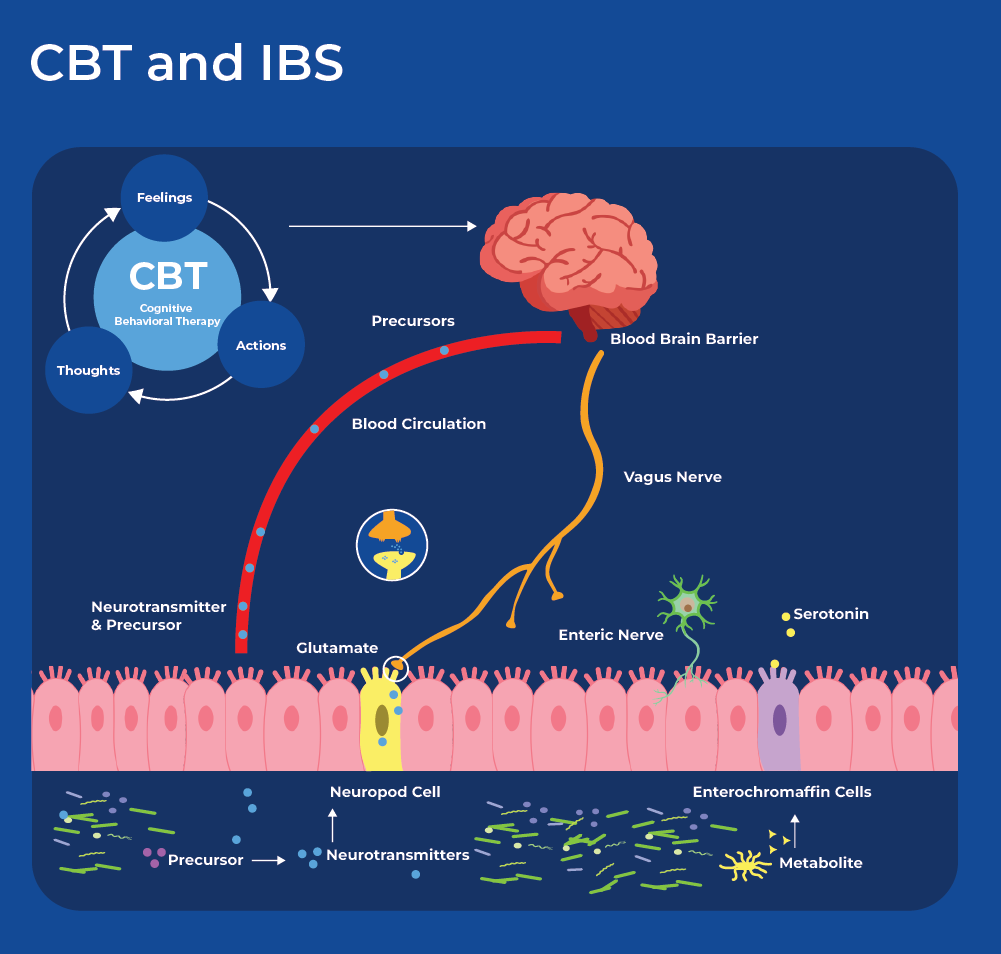
Cognitive Behaviour Therapy for the Treatment of IBS
Cognitive behavioral therapy (CBT) is a prevalent type of psychotherapy (talk therapy) in which subjects attend sessions with a mental health counselor with a goal of minimizing or correcting negative perceptual biases. CBT is often used to treat depression, addiction, eating disorders and marital problems. In IBS, negative emotions can cause GI discomfort, and GI discomfort can result in negative emotions. Therefore, CBT tailored for people with IBS can focus on managing stress responses through adaptive coping strategies for negative thoughts and emotions.
Recent research showed that cognitive behavioral therapy improved IBS symptoms through bidirectional alterations along the brain-microbiome axis associated with changes in microbial composition, microbial metabolites, structural connectivity of their brain networks and gastrointestinal symptom improvement.27 After CBT sessions 70% of subjects responded to the treatment with significantly decreased IBS symptoms (responders), while 30 % did not have significant reduction in symptoms (non-responders). Participants in the study underwent brain imaging, and had their gut microbiome sequenced as well as metabolites extracted and analyzed. Comparison of the responders to non-responders showed that they did not have any differences in their baseline gastrointestinal measurements between the groups, however they did have differences in their gut microbiome and fecal metabolites. Responders had increased abundance of Clostridales and decreased abundance of Bacteroides. Moreover, a classifier based on the abundance of 11 microbial genera was able to predict the treatment outcome. Interestingly, metabolomics profiling showed that fecal serotonin was significantly higher in responders than non-responders. Together these results suggest that microbial signals to the brain in the form of metabolites could modulate the responsiveness to CBT. Following treatment, neuroimaging revealed that CBT responders had changes in functional and structural connectivity of their brain networks as well as expansion of the Bacteroides groups.27
Related Norgen Products
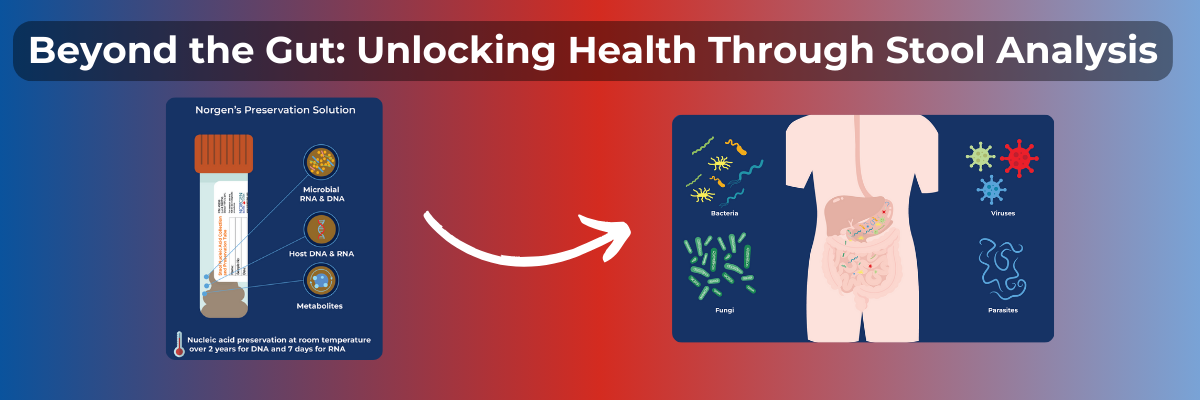
Norgen provides an entire workflow starting from preservation of stool through extraction of nucleic acids as well as NGS sequencing. Our preservation kits make sampling easy and keep DNA and RNA stable for 2 years and 7 days respectively. We have extraction kits for DNA, RNA and Nucleic Acids in a variety of formats (spin columns, 96-well plates and magnetic beads). The preserved stool is also compatible with metabolite analysis. Norgen also offers metagenomic library preparation kits, and associated products.
FAQs
Irritable Bowel Syndrome (IBS) and Inflammatory Bowel Disease (IBD) are two distinct gastrointestinal conditions, although they share some similar symptoms. IBS is a functional gastrointestinal disorder characterized by problems related to intestinal motility (bowel movements) and sensitivity (pain) resulting in either diarrhea or constipation and pain. On the other hand, IBD is an autoimmune condition characterized by chronic inflammation of the gastrointestinal tract, which results in tissue damage and complications such as ulcers, strictures, and fistulas. There are two main types of IBD: Crohn's disease and ulcerative colitis. Crohn's disease can affect any part of the digestive tract, while ulcerative colitis primarily affects the colon and rectum. IBD shares some symptoms with IBS such as abdominal pain, diarrhea (sometimes bloody), rectal bleeding, weight loss, however individuals with Crohn's disease may experience symptoms such as mouth sores, perianal disease, and inflammation of other parts of the body outside the digestive tract.