What is Cancer Immunotherapy?
Cancer is a term for a group of heterogeneous diseases in which cells grow uncontrollably and can eventually spread throughout the body. Traditional first-line treatment of cancer includes localized treatments such as radiation or surgery to physically remove cancerous cells, or systemic treatment such as chemotherapy. Chemotherapy involves systemic administration, usually through the bloodstream, of chemicals that kill rapidly growing cells most often through interfering with cell division. Although effective, Chemotherapy can result in resistance and very severe side effects. Many of these side effects are due to the fact that other non-cancerous fast growing cells can also be killed. New biological therapies are being introduced with a hope to reduce side effects and increase survival. Immunotherapy is a systemic biological treatment that uses a patient's own immune system to fight cancer. Three main types of immunotherapy are: Immune Checkpoint Inhibitors, T-cell transfer Therapy, and Monoclonal Antibodies. The gut microbiome influences the effectiveness of immunotherapy mainly through regulating the innate and adaptive immunity either directly or through bacterial metabolites.
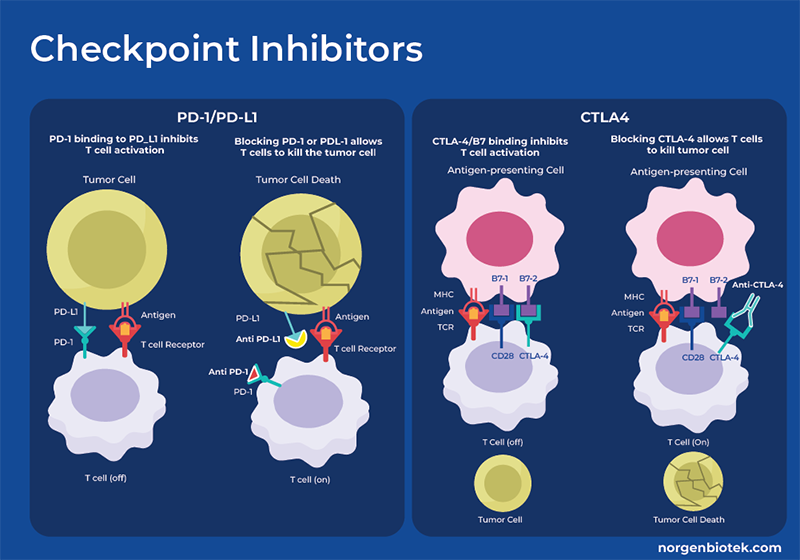
What are Checkpoint Inhibitors?
Immune Checkpoints are pathways that regulate the immune system and turn immune cells on and off. There are stimulatory checkpoint pathways that activate naive T-cells, as well as effector, memory and regulatory T-cell responses to generate an immune response. There are also inhibitory checkpoint molecules that are involved in keeping the immune system in check by dampening the immune response to help it discriminate between self and non-self cells/factors. Inhibitory checkpoint proteins on the surface of T cells, recognize and bind to complementary proteins on other cells, including certain tumor cells. Once the checkpoint and its partner proteins bind, they transmit an inhibitory signal to the T- cells, halting its activity, thus stopping them from attacking the cancer cell. These checkpoint proteins are critical in self-tolerance, as they prevent the immune system from attacking cells indiscriminately. However, some cancers can protect themselves from attack by stimulating immune checkpoint targets.
Immune Checkpoint Inhibitor (ICI), or Immune Checkpoint Blockade (ICB), therapy involves the administration of small molecules that block these inhibitory checkpoints and allow the immune cells to respond more strongly to cancer cells. One example is drugs that target the Programmed Death 1 (PD-1) receptor or its ligands, PD-L1 and PD-L2, thus allowing killing of the cancer cells. Another inhibitory checkpoint involves CTLA-4, short for Cytotoxic T-Lymphocyte-Associated protein 4, or CD152 (Cluster of Differentiation 152). While CTLA-4 is consistently present in regulatory T cells, its expression in conventional T cells increases only upon activation, as occurs in cancer. When bound to CD80 or CD86 on the surface of antigen-presenting cells, CTLA-4 functions as an "off" switch.
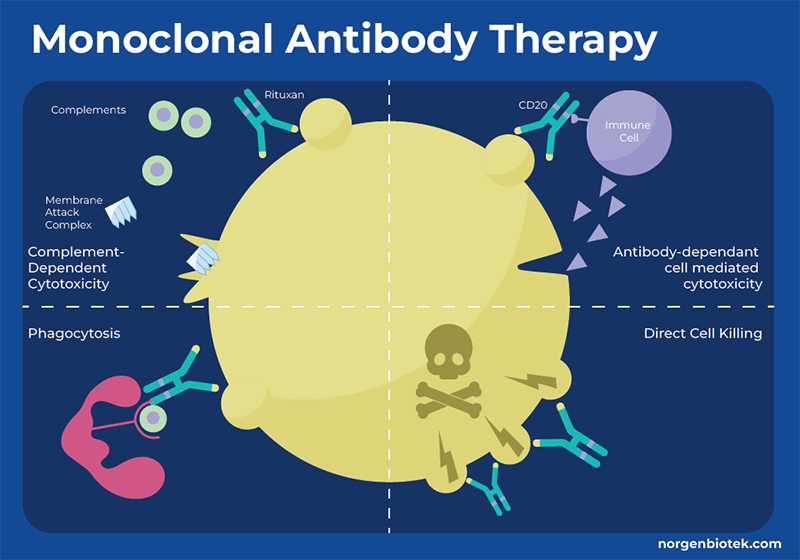
What is Monoclonal Antibody Therapy?
One approach by which the immune system identifies and eliminates threats is through antibodies. Antibodies bind to specific molecules (antigens) on the surface of target cells, such as bacteria, viruses or cancer cells. When an antibody attaches to an antigen, it acts as a signal to attract immune molecules triggering processes that lead to cell destruction by other immune mechanisms. However, cancer cells often evade detection by the immune system. They can employ strategies to conceal themselves or release signals that impede the proper functioning of immune cells. Monoclonal antibodies (mAbs) are proteins that are generated in the laboratory to bind to a specific antigen, thus enhancing or mimicking the immune system's assault on undesired cells, such as cancer cells. Monoclonal antibodies can function through a variety of mechanisms, and many monoclonal antibody drugs may employ multiple functions. These functions can include flagging cancer cells for detection and destruction, triggering cell-membrane destruction, blocking cell growth. Each monoclonal antibody targets a specific protein on the cancer cell, therefore different mAbs must be created for different types of cancer or different target molecules for even the same type of cancer.
What is T-Cell Transfer Therapy?
T-cell transfer therapy is another type of immunotherapy, however in this instance a patient’s own immune cells are transformed to better target and kill cancer cells. There are two main types of T-cell transfer therapy: tumor-infiltrating lymphocytes (or TIL) therapy and CAR T-cell therapy. In TIL therapy, doctors test the lymphocytes that are found in your tumor to determine which are best at recognizing the tumor. These are then selected, grown rapidly and injected back into the patient. CAR T-cell therapy is similar to TIL therapy, but the patient's T-cells are genetically modified before they are grown and re-introduced into the patient. As T-cell receptors may not always recognize cancerous cells, these T-cells are modified to produce a specific chimeric antigen receptor (CAR) that is designed to allow the T cells to attach to specific proteins on the surface of the cancer cells, improving their ability to attack the cancer cells. Once infused into your bloodstream, CAR T-cell receptors locate and eliminate cancerous cells. Furthermore, these cells continue to proliferate, ensuring a sustained reservoir of CAR T-cells targeting your cancer cells.
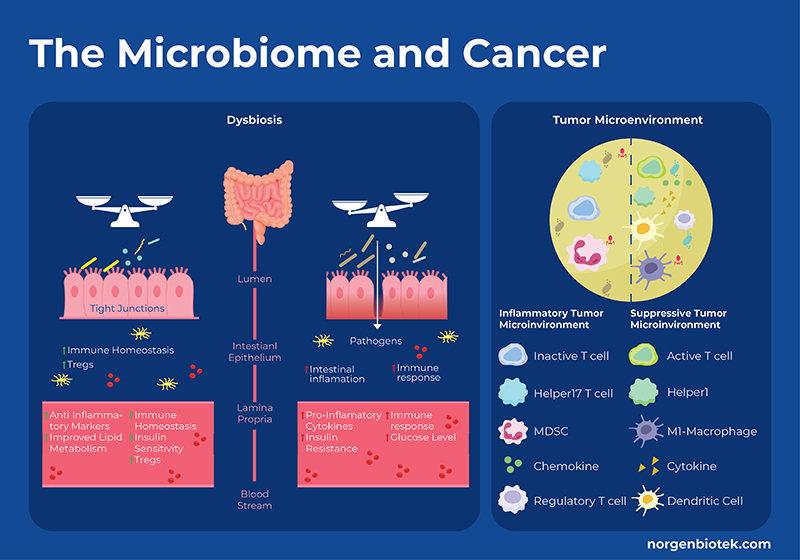
How Does the Microbiome Influence Cancer?
The gut microbiome can both stimulate or suppress cancer progression. Detrimental bacteria can produce toxic metabolites, suppress the immune system, inhibit apoptosis, and encourage inflammation, all promoting cancer progression. Whereas, beneficial bacteria can produce metabolites such as short-chain fatty acids, immunomodulatory lipids 1, amino acids, or anti-inflammatory metabolites that can interact with the immune system and metabolic pathways related to cell growth and apoptosis. Specifically, fermentation of dietary fiber produces short chain fatty acids (SCFAs) that have been shown to control regulatory T cell populations through inhibition of host histone deacetylases (HDAC), and interactions with cell surface receptors. In addition to immune cells, butyrate has also been shown to modulate the release of inflammatory cytokines, and production of tumor necrosis factor alpha. On the other hand, microbial metabolism of dietary cholesterol produces secondary bile acids that can be converted by bacteria into cytotoxic compounds that increase colonic cell proliferation. Dysbiosis occurs when the proportion of ‘bad’ bacteria outweighs the proportion of ‘good’ bacteria and is generally accompanied by a reduction in overall bacterial diversity. Dysbiosis has been shown to be involved in cancer progression through a few mechanisms including the enrichment of protumorigenic macrophages, hyperactivation of CD8+ T cells, driving them into exhaustion to name a few.
Moreover microbes in the tumor microenvironment (TME) have also been shown to modulate cancer progression. Recent research suggests that microbes inhabit both tumor cells and immune cells. There are a variety of mechanisms why which microbes in the TME can influence immunotherapy such as: 1) cancer cells and immune cells bearing microbial antigens 2) microbial induction of immunogenic cell death, thus eliciting an inflammatory TME 3) microbes stimulating or repressing an immune response through pattern recognition receptors, thus altering the balance of different immune cells 4) through the action of microbial metabolites as discussed above 5) microbial stimulation of inhibitory checkpoints. For example, Fusobacterium nucleatum is implicated in several cancers such as colon cancer and often detected in the TME. It has been shown that the F. nucleatum outer-surface protein Fap2 binds to and activates the human inhibitory receptor TIGIT, which is expressed by T and natural killer (NK) cells, thereby suppressing anti-tumor immunity. Moreover, F. nucleatum also binds to and activates the human inhibitory receptor CEACAM1, resulting in the inhibition of T and NK cell activities. 2 Conversely, probiotic bacteria like Bifidobacterium upregulate the expression of Type 1 interferons (IFN-I) in dendritic cells via the stimulator of interferon gene (STING) signaling pathway. This promotes antigen cross-presentation and T-cell activation, thereby enhancing the efficacy of CD47 blockade. 3
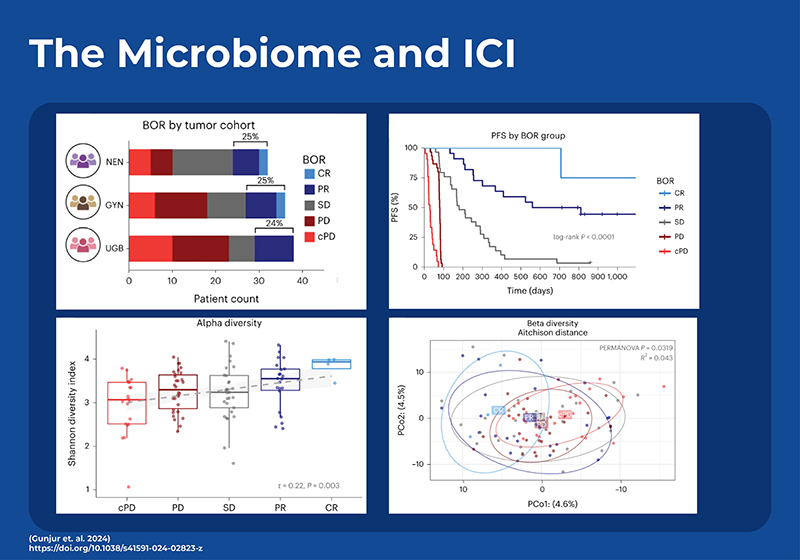
How Does the Microbiome Influence Checkpoint Inhibitor Therapy?
Checkpoint inhibitor therapy has proven to be an effective treatment therapy that can increase overall survival rates, however there is considerable heterogeneity in the response with up to 10 - 67 % not responding depending on the type of cancer and treatment regime 4,5. Studies are now showing that the microbiome plays a key role in determining if a patient responds to immunotherapy as well as if they have side-effects from toxicities. The presence (and frequency) or absence of particular bacterial strains before treatment may influence the response to, and side effects of, immune checkpoint inhibitors.
The first study in human patients to bring this to light was an anti-PD1 trial with melanoma patients. 6 NGS metagenomic sequencing of the patients fecal microbiome showed that responders had significantly higher microbial diversity as well as higher relative abundance of Clostridiales including multiple Feacalibacterium strains. The responders also had increased frequencies of effector CD4+ and CD8+ T -cells in the systemic circulation, along with a sustained cytokine response to anti-PD-1 therapy. Conversely, patients with a higher prevalence of Bacteroidales in their gut microbiome, non-responders, showed higher frequencies of Tregs and myeloid-derived suppressor cells (MDSCs) in the systemic circulation, accompanied by a diminished cytokine response. To further confirm these results they performed Fecal Microbiome Transplants (FMT) using the stool of anti-PD1 responders (R-FMT) or non-responders (NR-FMT). The mice that received a FMT with stool from responders, had significantly reduced tumor size and exhibited improved responses to anti–PD-L1 therapy in contrast to mice that were transplanted with stool from non-responders. Tumors of mice receiving R-FMT stool had a higher density of CD8+ T cells than mice receiving NR-FMT. Since this study there have been several others showing that specific microbiome composition signatures can predict patient response to treatment with antibodies against programmed cell death protein (PD)-1, or its ligand PD-L1, and cytotoxic T lymphocyte-associated protein (CTLA)-4, across several solid tumors.
To date, microbial signatures found to be predictive of response in one study haven’t had great predictive power on other external data sets. In an effort to see if a generalized signature for ICI response can be elucidated, a recent study employed temporal, in-depth shotgun metagenomic profiling in a cohort of phase 2 trial patients with three diverse rare cancers treated with combination ipilimumab (anti-CTLA-4) plus nivolumab (anti-PD-1).7 Most previous studies had used 16S sequencing as opposed to shotgun sequencing, however many microbial characteristics vary at the strain level and may not be detected by 16S. This new study also found a positive relationship between the gut microbiome diversity and improved response, and identified a signature of 22 gut microbial strains that could predict which patients would respond to the ICI therapy. Analysis of this strain-based model showed that the bacteria with the most predictive power was an uncultured strain of Faecalibacterium (sp900539885). Although the model performed better when trained separately on each cancer type, it still performed well when generalized across all three cancer types. To evaluate the external generalizability of the model they reanalyzed all comparable shotgun metagenomic cohorts. When examining the metadata they determined that the two dominant factors affecting microbial variance across the meta-cohort were study site (city) (9.3%) and DNA extraction kit (8.0%). Although the predictive power was less than required for clinical use, they demonstrated that the strain-level predictor could only be generalized across cancer types, and study location when the model was built using data from concordant treatment regimes.This indicates that gut microbiome diagnostics or therapeutics should be customized based on the regimen of immune checkpoint blockade (ICB) treatment rather than solely based on the type of cancer.
In order to improve the clinical applicability of using the microbiome as a biomarker of efficacy and toxicity in cancer patients receiving immune checkpoint inhibitor therapy, a large clinical trial is underway. The MITRE (Microbiome Immunotherapy Toxicity and Response Evaluation) study funded by Microbiotica and Cancer Research UK has greatly increased the sample size, and provides for more rigorous and standardized methods. It involves up to 40 UK sites, and 1800 cancer patients as well as 360 household control participants. The patients are split into cohorts based on the type of cancer, the stage of cancer, and a variety of treatment regimes including anti-PD1, anti-CTLA-4, anti-PD(L)1 + kinase inhibitor, as well as different immunotherapy combinations and combinations with chemotherapy. Stool, and oral swabs will be collected, and whole genome shotgun metagenomics will be performed to determine the correlation of both oral and fecal microbiomes with treatment efficacy. In addition, radiological assessment of tumors, blood and biopsies will be collected and tested for a vast array for immunological, and genomic testing. They will obtain peripheral blood immune and cytokine profiles, genomic or transcriptomic alterations in blood (including germ-line) and tumors, as well as any cell-free circulating DNA (cfcDNA). Two additional intriguing exploratory outcomes include the use of specialized microbial isolation and cultivation methods to isolate and preserve individual bacterial strains, thereby enabling hypothesis testing with the identified organisms. Additionally, they intend to create primary cell lines from patient peripheral blood mononuclear cells (PBMCs) and tumor tissue for conducting functional immune assays.
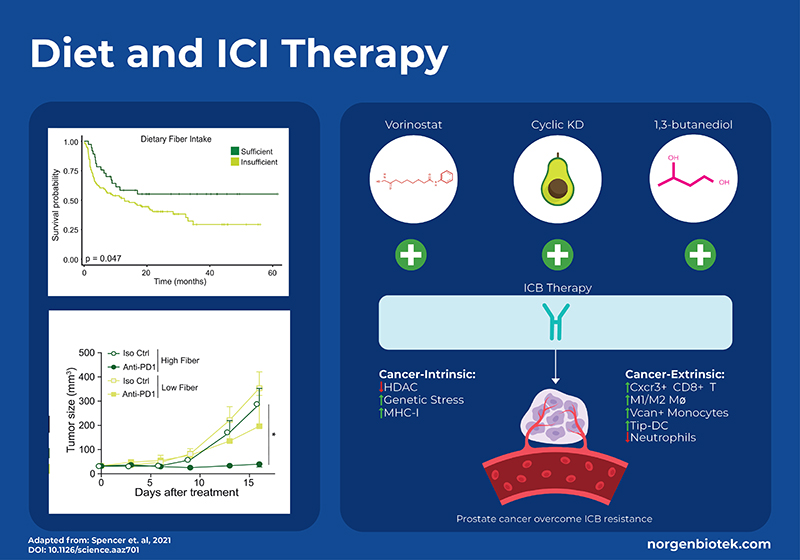
The Effect of Diet on Immunotherapy
High Fiber Diet and Immunotherapy
Diet is a modulator of the gut microbiome. Bacteria digest food and transform cholesterol into secondary bile acids, metabolize amino acids to immunomodulatory lipids 1, and ferment fiber into short-chain fatty acids (SCFA). Diets that are high in fiber have been shown to have positive effects on health. The consumption of fiber aids in the growth of SCFA producing microbes which has been shown to be important for regulating the immune system 8. To understand how a high-fiber diet can affect the outcome of immunotherapy, a large clinical trial for melanoma patients documented their fiber intake. Increased consumption of dietary fiber, 20g/day or higher, was linked to enhanced progression-free survival among 128 patients undergoing ICB treatment. Moreover, stool-based metagenomic sequencing showed that responders had microbial profiles more similar to each other and had significantly higher levels of SCFA-producing bacteria (Ruminococcus & Faecalibacterium). Interestingly, consumption of over-the-counter probiotics decreased the overall diversity and did not result in increased survival. To further test these results, mouse models of melanoma were fed either a high-fiber or standard diet and treated with anti–PD-1 therapy. The mice that received the high-fiber diet had significantly less tumor growth. To demonstrate that the dietary effect is through modulating the microbiome, it was shown that there was no difference in tumor growth between high and low fiber diets in germ-free mice. Immune cell profiling of the tumor revealed increased CD4+ T cells, including those expressing PD-1, in mice fed high-fiber diets compared to those on low-fiber diets. Additionally, RNA sequencing of CD45+ tumor-infiltrating lymphocytes (TILs) demonstrated higher expression of genes associated with T-cell activation and interferon response in mice receiving high-fiber diets alongside anti-PD-1 treatment, in contrast to those on low-fiber diets. 9
Ketogenic Diet and Immunotherapy
Another diet that has been associated with positive health outcomes is the ketogenic diet (KD). The ketogenic diet focuses on high-fat intake and low carbohydrate intake. This encourages the body to use fat, as opposed to glucose, for energy generation. On a ketogenic diet, fatty acid oxidation occurs in the liver resulting in the production of ketone bodies such as β-hydroxybutyrate (BHB). It is known that BHB acts as a signaling metabolite regulating gene expression and cellular function, and is an endogenous inhibitor of histone deacetylase (HDAC) enzymes. Histone deacetylases (HDACs) play an important role in cellular function and have been shown to exert diverse immunomodulatory effects and directly impact cancer cells. Studies have shown that the ketogenic diet enhances the effectiveness of PD-1 or CTLA4 antibodies in models of melanoma, renal cancer, and colon cancer. 10,11 However, a recent study looked into the molecular mechanics of this in relation to prostate cancer which has one of the lowest response rates to immunotherapy.12
Murphy et al., generated a mouse model of prostate cancer with acquired ICI resistance. They compared monotherapy with either anti-PD1 and anti-CTLA4 to combination therapy with the addition of an HDAC inhibitor (vorinostat), a ketogenic diet, or supplementation of the ketone body β-hydroxybutyrate (BHB, an endogenous HDACi) via 1,3-butanediol. Results showed that a cyclic ketogenic diet produced better synergistic effects than a continuous ketogenic diet, moreover supplementation with 1,3- butanediol had even better anti-cancer effects. In addition to measuring survival and tumor growth they used single-cell transcriptomics (scRNA-seq) and proteomics, as well as flow cytometry sorting of immune cells. scRNA-seq showed that 1,3-butanediol supplementation upregulated CD8+ T cells and specific myeloid populations in the tumor microenvironment. Overall, this study shows that a cyclic ketogenic diet, or supplementation with 1,3 butanediol can improve the outcome for ICI-reistant therapy through processes that are intrinsic to the cancer cells (ie. upregulation of MHC molecules) as well as extrinsic immune functions such as attraction of CD8+ T cells, differentiation of antigen presenting cells and reduced neutrophils. 12
This study used Norgen Biotek’s FFPE RNA Purification Kit (Cat. 25360) to extract RNA from FFPE biopsy samples for subsequent qRT-PCR to monitor expression of PD-L1, CTLA-4, Granzyme-B and other relevant genes.
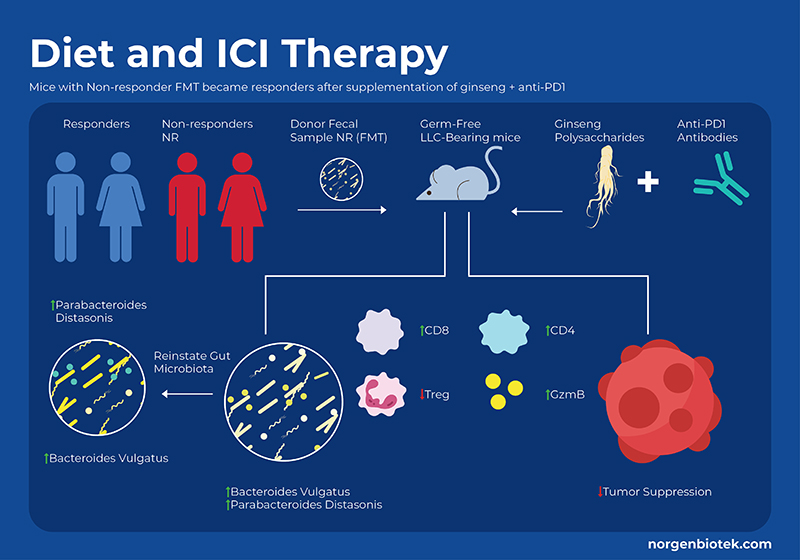
Herbal Supplements and Immunotherapy
For millennia, Panax ginseng has been used as a medicinal remedy and as a dietary supplement. It has been shown to modulate the gut microbiome, and provide many health benefits. Ginseng contains a variety of bioactive molecules including phenolic compounds, ginsenosides, essential oils, peptidoglycans, nitrogen-containing compounds, fatty acids, and polysaccharides. Studies have shown that ginseng polysaccharides (GPs) not only increase the proportion of beneficial probiotic bacteria, but also have immunomodulatory functions, such as the activation of macrophages, T cells and natural killer cells. Researchers in a recent study used a mouse model of lung cancer to examine the effect of supplementing anti-PD-1 treatment with GP extract. They found that supplementation with GPs decreased tumor size,and increased survival rate. They also used flow cytometry to analyze systemic immunological cell profiles (blood and spleen) as well as local changes in the tumor. The combination of GP+ICI treatment increased the CD8+/CD4+ ratio as well as an increase in functional cytokines, and downregulation of Treg cells. 13
By measuring metabolites they observed that cotreatment increased SCFA concentrations, specifically valerate, which is not normally high. Interestingly, research suggests that valerate (pentanoate) is an HDAC inhibitor14. In addition to SCFAs, certain amino acids have been shown to be involved in the response to immunotherapy, specifically the tryptophan pathway. The enzyme Indoleamine 2,3-dioxygenase (IDO) catalyzes the conversion of tryptophan (Trp) to kynurenine (Kyn). In the TME, IDO suppresses the functions of T-cells while promoting generation and activation of Tregs and MDSCs. Previous studies have shown that IDO inhibitor drugs combined with anti-PDL1 and anti-CTLA4 antibodies suppressed cancer growth through enhanced proliferation and infiltration of cytotoxic T lymphocytes (CTLs) and Interleukin-2 (IL-2) production in preclinical models.15 This study showed the ginseng polysaccharides combo therapy led to a reduced Kyn/Trp ratio (the outcome of IDO inhibition) as compared to anti-PD1 alone. They further confirmed this result through in vitro fermentation of human faecal samples from healthy donors with GPs and found an increase in tryptophan and a reduction in kynurenine levels under both aerobic and anaerobic conditions.In conclusion supplementation of ginseng polysaccharides combined with anti-PD1 treatment improved cancer prognosis through altering the immunological profile and metabolite levels.
Summary
ICI is a promising cancer treatment currently limited by variable response rates. Metagenomic analysis of the stool microbiome represents a non-invasive method to detect biomarkers of ICI response and toxicity. Moreover, modulation of the human gut microbiome through administration of specific bacterial strains or through diet may prove to help increase the response rate and effectiveness of immunotherapy. Several clinical trials are underway to test the administration of specific live therapeutic probiotics as well as dietary regimes.