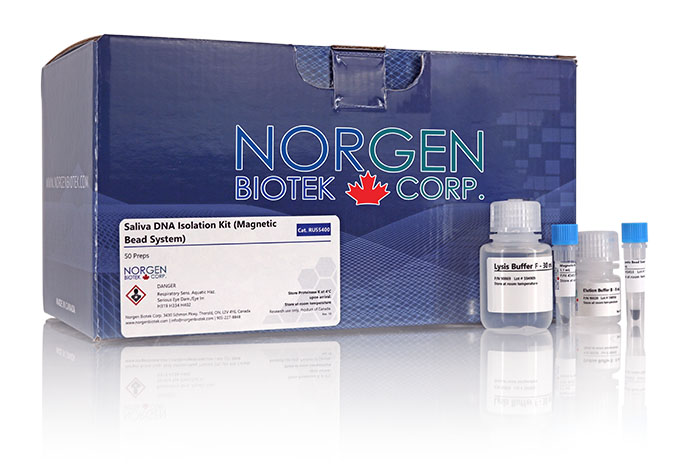
Saliva DNA Isolation Kit
For the rapid purification of high-quality DNA from preserved and fresh saliva samples



Saliva DNA Isolation Kit
For the rapid purification of high-quality DNA from preserved and fresh saliva samples
Register today to receive an exclusive 15% off* on your first order.
Features and Benefits
- Fast and easy processing using a rapid spin-column format
- Isolate high quality genomic DNA from fresh or preserved saliva samples
- Compatible with preserved saliva samples collected using Norgen’s Saliva DNA Collection and Preservation Devices (Cat. RU49000)
This kit provides a fast and simple spin column procedure for isolating genomic DNA from saliva samples collected and preserved using Norgen’s Saliva DNA Collection and Preservation Devices (Cat. RU49000), as well as fresh saliva samples.
Saliva DNA purified using Norgen’s kit is of the highest quality, and is compatible with a number of downstream research applications including PCR, Southern Blot analysis, sequencing and microarray analysis.
Background
Saliva represents an excellent non-invasive alternative to blood collection. Human genomic DNA extracted from buccal epithelial cells and white blood cells found in saliva can be used in various applications in diagnostics. Saliva DNA can be used for the detection of biomarkers to diagnose a disease, follow the diseases progress or monitor the effects of a particular treatment. Saliva DNA can also be used to diagnose particular types of infections. Isolation of DNA from saliva has become an attractive alternative to isolation from blood or tissue due to the fact that sample collection is non-invasive, the samples can be collected by individuals with little training, and no special equipment is required. Norgen’s Saliva DNA Isolation Kit provides a fast and simple procedure for isolating genomic DNA from both preserved saliva samples and fresh saliva samples.
Details
Supporting Data
Figure 1. High Quality and Yield of DNA from Saliva Samples. Total DNA was isolated from 250 µL of eight different fresh saliva samples using Norgen's Saliva DNA Isolation Kit (Lanes 1-8). For evaluation, 10 µL of each 100 µL DNA elution was run on a 1.2 % agarose gel. Note the high yield and quality of the DNA in all lanes. Lane M: Norgen's Fast runner 1kb DNA Ladder.
Figure 2. Real-time PCR Consistency from Saliva Samples. Norgen's Saliva DNA Isolation Kit was used to isolate DNA from 250 µL samples of fresh saliva. Ten samples were used from same donor. From the 100 µL elution, 5 µL of saliva DNA was directly mixed to real-time PCR master mix (total reaction volume 20 µL), and the real-time PCR reaction was performed. The GAPDH gene was successfully amplified from the different samples without any inhibition and at the same Ct, indicating the isolation consistency and the excellent quality of saliva DNA for downstream applications.
Figure 3. Illumina MiSeq 16s rRNA metagenomics data from saliva samples preserved for over 6 years using Norgen's Saliva DNA Collection and Preservation Devices. The saliva DNA was isolated using Norgen's Saliva DNA Isolation Kit (Cat. RU45400) from saliva that had been preserved for various periods of time up to 6 years at room temperature. A) Principal Coordinate Analysis of 12 saliva microbiomes showing differences in the distribution of taxonomic classifications between samples up to kingdom level. B) Hierarchical clustering of 12 saliva microbiomes based on genus-level classifications, including a bar chart showing the relative abundance of genus-level classifications for each sample in the dendrogram.
|
Kit Specifications
|
|
|
Maximum Saliva Input
|
0.5 mL preserved saliva
0.25 mL fresh saliva |
|
Average Yield from 0.25 mL of Saliva*
|
3 - 7 μg
|
|
Average Purity (OD260/280)
|
1.7 - 2.1
|
| Time to Complete 10 Purifications |
30 minutes
|
* Average yield will depending on the donor
Storage Conditions and Product Stability
All solutions should be kept tightly sealed and stored at room temperature. This kit is stable for 1 year after the date of shipment. The kit contains a ready-to-use Proteinase K, which is dissolved in a specially prepared storage buffer. The buffered Proteinase K is stable for up to 1 year after the date of shipment when stored at room temperature.
| Component | Cat. RU45400 (50 preps) |
|---|---|
| Lysis Buffer F | 30 mL |
| Proteinase K in Storage Buffer | 1.2 mL |
| Binding Buffer B | 12 mL |
| Wash Solution A | 18 mL |
| Elution Buffer B | 15 mL |
| Spin Columns | 50 |
| Collection Tubes | 50 |
| Elution Tubes (1.7 mL) | 50 |
| Product Insert | 1 |
Documentation
The Range of DNA Yield with Norgen’s Saliva DNA Collection, Preservation and Isolation Kit
Determination of the DNA Molecular Weight (MW) from different Norgen Columns and Isolation Methods
Stability of DNA Stored in Norgen’s Saliva DNA Preservative for 52 Months at Room Temperature
Linearity of DNA Isolated from Increasing Volumes of Preserved Saliva Using Norgen’s Saliva DNA Isolation Kit
DNA Isolation from Saliva Preserved with Norgen’s Saliva DNA Collection and Preservation Device using Qiagen’s QIAamp DNA Blood
Comparative Study of DNA Isolated from Saliva Preserved in Norgen’s Preservative Using Norgen’s Saliva DNA Isolation Kit Versus
The Effect of Elution Volume on DNA Quantity and Quality Using Norgen’s Saliva DNA Isolation Kit
A Comparative Study Between Two Saliva DNA Preservation Systems, and Two Column-Based Saliva DNA Purification Methods
Column-Based Isolation of DNA from Preserved Saliva Samples Offers High Quality, Consistent, and Rapid DNA Isolation
Stability of DNA Stored in Norgen’s Saliva DNA Preservative at 55°C
Long Term Stability of DNA Stored in Norgen’s Saliva DNA Preservative
FAQs
Spin Column
Column clogging may occur due to one or combination of the following factors:
- Centrifugation speed was too low or spin time was inadequate.
Check the centrifuge to ensure that it is capable of generating the required RPMs. Sufficient centrifugal force is required to move the liquid phase through the column. Also ensure that the correct spin times are followed. Spinning for a few additional minutes will help. - The sample is too large.
Too many cells were applied to the column. Ensure that no more than 0.5 mL of preserved saliva is applied to the column. Clogging can be alleviated by centrifuging for a longer period of time until the lysate passes through the column. - The lysate/binding solution mixture is not homogeneous.
To ensure a homogeneous solution, vortex for 10- 15 seconds before applying the lysate to the spin column.
Low genomic DNA yield may result from one or combination of the following factors:
- Incomplete lysis of cells Increased.
Proteinase K incubation time at 55°C may result in increased yields. -
The DNA elution is incomplete.
Perform an additional centrifugation of 2 minutes at 14,000 x g to ensure that all the DNA is eluted. -
DNA concentration in the saliva sample being used is low.
Some saliva samples contain very little DNA. This varies from individual to individual based on numerous variables. Increased proteinase K incubation time at 55°C may result in increased yields.
Poor downstream performance of DNA may result from one or combination of the following factors:
-
DNA was not washed with Wash Solution A.
Traces of salt from the binding step may remain in the sample if the column is not washed with Wash Solution A. Salt may interfere with downstream applications, and thus must be washed from the column. -
Ethanol carryover.
Ensure that the dry spin after the column wash steps is performed, in order to remove traces of ethanol prior to elution. Ethanol is known to interfere with many downstream applications.
RNA might get co-eluted with DNA in some cases. Carry out a digestion with RNase A on the elution if the RNA present will interfere with downstream applications. Refer to manufacturer’s instructions regarding amount of enzyme to use, optimal incubation time and temperature.
Swab samples can be processed using Saliva DNA isolation kit following a supplementary proptocol. However, please note that swab samples are best processed using Norgen's Microbiome DNA isolation kit.
Yes, it has been tested on canine samples, and it is expected to work on most other non-human samples. Please contact our technical support team at support@norgenbiotek.com and ask for reference publications.
Yes, please contact our technical support team at support@norgenbiotek.com for help with supply of extra proteinase K in storage buffer.
Citations
| Title | Assessment of oral bacteria potentially associated with the mobile microbiome in children with congenital heart disease |
| Citation | Journal of Clinical Pediatric Dentistry 2024. |
| Authors | Sermin Dicle Aksakal1 , Yeliz Guven1,* , Nursen Topcuoglu2 , Guven Kulekci?2 , Oya Aktoren |
| Title | Genetic variants of the beta-adrenergic receptor pathways as both risk and protective factors for retinopathy of prematurity. |
| Citation | American Journal of Ophthalmology 2024. |
| Authors | Hélène Paradis Salem Werdyani Guangju Zhai Robert L. Gendron Reza Tabrizchi Margaret McGovern J. Michael Jumper Daniel Brinton William V. Good |
| Title | Pursuing dynamics of minimal residual leukemic subclones in relapsed and refractory acute myeloid leukemia during conventional therapy |
| Citation | Cancer Medicine 2024. |
| Authors | Dongchan Kim1,2 | Sheehyun Kim2,3 | Hyojin Song2,3 | Daehyeon Gwak1,2 | Suji Min1,2 | Ja Min Byun1,2,4 | Youngil Koh1,2,4 | Junshik Hong1,2,4 | Sung-Soo Yoon1,2,4 | Hongseok Yun2,3 | Dong-Yeop Shin1 |
| Title | UGT1A1*28 detection using high-resolution agarose gel electrophoresis |
| Citation | Heliyon 2024. |
| Authors | Shirou Tsuchida Takaaki Hirayama Hayato Nunose Hinako Suzuki Ryo Hakota Tsugumi Shindo Koji Nakagawa |
| Title | Effect of the Rs2923234 and Rs1049112 Single Nucleotide Polymorphisms of Salivary Acidic Proline-rich Protein on Dental Caries in Young Children: An Analytical Cross-sectional Molecular Study |
| Citation | Dental Hypotheses 2023. |
| Authors | Maitha Sameer Kadhim1, Ahlam Taha Mohammed2 |
| Title | Reduced microbial diversity of the nasopharyngeal microbiome in household contacts with latent tuberculosis infection |
| Citation | Scientific Reports 2023. |
| Authors | Cinthya Ruiz-Tagle, Juan A. Ugalde, Rodrigo Naves, Rafael Araos, Patricia García & María Elvira Balcells |
| Title | Sputum Microbiome and Chronic Obstructive Pulmonary Disease in a Rural Ugandan Cohort of Well-Controlled HIV Infection |
| Citation | Microbiology spectrum 2023. |
| Authors | Alex Kayongo , Theda Ulrike Patricia Bartolomaeus, Till Birkner, Lajos Markó, Ulrike Löber, Edgar Kigozi, Carolyne Atugonza, Richard Munana, Denis Mawanda, Rogers Sekibira, Esther Uwimaana, Patricia Alupo, Robert Kalyesubula, Felix Knauf, Trishul Siddharthan, Bernard S. Bagaya, David P. Kateete, Moses L. Joloba, Nelson K. Sewankambo, Daudi Jjingo, Bruce Kirenga, William Checkley, Sofia K. Forslund |
| Title | The Val158Met Polymorphism in 8-Year-Old Boys and Girls Moderates the Influence of Parenting Styles on Proactive Aggression: Testing the Sensitivity to the Environment |
| Citation | Brain sciences 2023. |
| Authors | Nora del Puerto-Golzarri, Aitziber Azurmendi, José Manuel Muñoz, María Rosario Carreras and Eider Pascual-Sagastizabal |
| Title | Factors associated with canine skin extensibility in toy poodles |
| Citation | The Journal of Veterinary Medical Science 2022. |
| Authors | Takeda M, Arai N, Koketsu Y, Mizoguchi Y. |
| Title | The myth of oral hygiene using synthetic mouthwash products |
| Citation | SpringerPlus 2016. |
| Authors | Jahangir, G. Z., Ashraf, D. S., Nasir, I. A., Sadiq, M., Shahzad, S., Naz, F., ... & Saeed, A |