RNA/Protein Purification Plus Kit
For sequential isolation of total RNA and total proteins from the same sample

For research use only and NOT intended for in vitro diagnostics.
Formerly Cat. 24100
RNA/Protein Purification Plus Kit
For sequential isolation of total RNA and total proteins from the same sample
Register today to receive an exclusive 15% off* on your first order.
Features and Benefits
- Sequentially purify total RNA and total proteins from a single sample
- Kit includes a gDNA elimination column
- No sample splitting required
- No phenol step required for efficient isolation
- Ideal for small or difficult to obtain samples
- Purify RNA and proteins from cultured animal cells, tissues, blood, bacteria, yeast, fungi or plants
- Rapid and efficient spin column procedure
- Purification is based on spin column chromatography that uses Norgen’s proprietary resin separation matrix
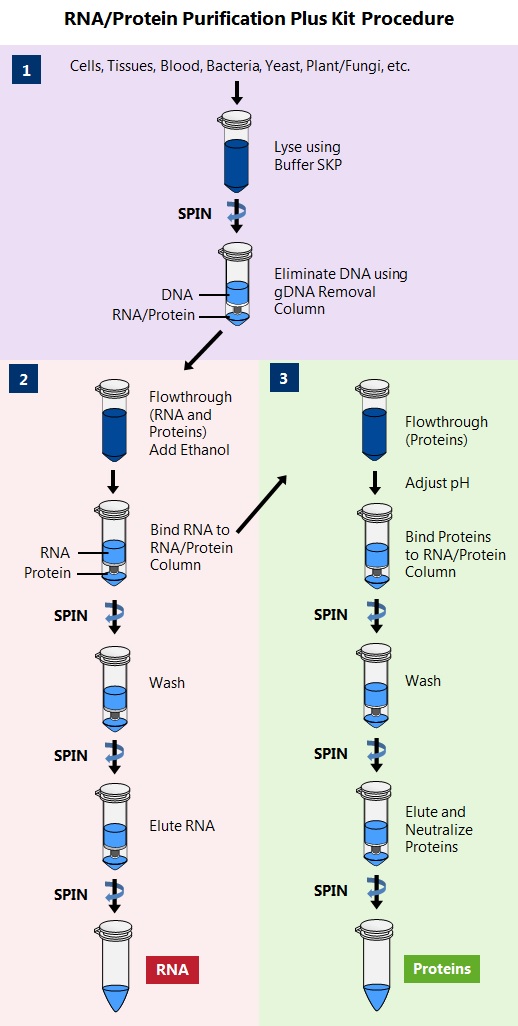
This kit provides a rapid, single column method for the isolation and purification of total RNA (including miRNA) and proteins sequentially from a single sample of cultured animal cells, tissues, blood, bacteria, yeast, fungi or plants. The total RNA and proteins are both column purified in under 25 minutes using a single column.
Purified RNA is of a high quality and yield, and is suitable for NGS, RT-qPCR and microarrays.
This kit eliminates DNA efficiently using a gDNA removal column.
Proteins are eluted in buffer and are ready for downstream applications such as Western Blots, Mass Spec and ELISA. The proteins will not require precipitation, resuspending of pellets, or any further cleaning.
This kit is ideal for researchers who are interested in studying the transcriptome and proteome of a single sample, such as for studies of microRNA profiling, gene expression including gene silencing experiments or mRNA knockdowns, studies involving biomarker discovery, and for characterization of cultured cell lines. Norgen’s RNA/Protein Purification Plus Kit is especially useful for researchers who are isolating macromolecules from precious, difficult to obtain or small samples such as biopsy materials or single foci from cell cultures, as it eliminates the need to fractionate the sample. Furthermore, analysis will be more reliable since the RNA and proteins are derived from the same sample, thereby eliminating inconsistent results. The purified macromolecules are of the highest purity and can be used in a number of different downstream applications.
Protocol
Details
Supporting Data
Figure 1. Recovery of True Total RNA including microRNA from Hamster Liver. Panel A is a 1X MOPS 1% agarose gel showing the RNA that was isolated from 2 different samples of 10 mg hamster liver using Norgen's RNA/Protein Purification Plus Kit. Norgen's RNA/Protein Purification Plus Kit isolated both large RNA (represented by 28S and 18S rRNA) as well as small RNA with high integrity and without having to perform any additional protocol. Panel B is a result from a bioanalyzer resolution of the eluted RNA. Similar to the agarose gel, the Bioanalyzer showed that Norgen's RNA/Protein Purification Plus Kit has the added benefit of recovering small RNA. One microgram of the RNA was used in RT-qPCR reactions for the detection of human beta-Actin (for Large RNA) and miR-21 (for microRNA) genes. The RNA isolated using Norgen's RNA/Protein Purification Plus Kit showed superior recovery of both large RNA and small RNA including microRNAs as depicted by the successful miR-21 RT-qPCR (Panel C).
Figure 2. High Quality Total Proteins Eluted in Mass Spec-Compatible Buffer. Norgen's RNA/Protein Purification Plus Kit provides a column purification step for effective concentration and clean-up of the isolated proteins. The proteins are eluted into a buffer which is compatible with many downstream applications including mass spectrometry as well as standard protein quantification methods (including Bradford assays). In contrast, most competing multiple analyte isolation products require protein precipitation and the precipitated proteins are required to be resuspended in buffer with high-detergent content (such as SDS-PAGE loading dye) for full recovery. In this figure, the protein fraction isolated from hamster liver using Norgen's RNA/Protein Purification Plus Kit was resolved direclty on a 12% SDS-PAGE protein gel. Norgen's RNA/Protein Purification Plus Kit purified the proteins by column and the eluted proteins are already in buffer compatible with downstream applications.
|
Kit Specifications
|
|
| Maximum Column Binding Capacity | 50 μg for RNA |
| Maximum Column Binding Capacity | 200 μg for Protein |
| Maximum Column Loading Volume | 650 μL |
| Size of RNA Purified | All sizes, including small RNA (< 200 nt) |
| Time to Complete 10 Purifications | 30 minutes |
| Average Yields*: HEK 293 Cells (1 x 106 cells) HEK 293 Cells (1 x 106 cells) Liver (15 mg) Liver (15 mg) |
10-15 μg RNA 70-100 μg protein 30-35 μg RNA 100-150 μg protein |
* Average yields will vary depending upon a number of factors including species, growth conditions used and developmental stage.
Storage Conditions and Product Stability
The Protein Loading Dye should be stored at -20°C after the addition of DL-Dithiothreitol (DTT). All solutions should be kept tightly sealed and stored at room temperature. This kit is stable for 1 year after the date of shipment.
Component | Cat. 48200 (50 preps) |
|---|---|
Buffer SKP | 40 mL |
Wash Solution A | 38 mL |
Elution Solution A | 6 mL |
Wash Solution C | 30 mL |
Binding Buffer A | 8 mL |
Elution Buffer C | 4 mL |
Protein Neutralizer | 4 mL |
Protein Loading Dye | 2 mL |
gDNA Removal Columns | 50 |
RNA/Protein Purification Columns | 50 |
Collection Tubes | 150 |
Elution Tubes (1.7 mL) | 100 |
Product Insert | 1 |
Documentation
FAQs
Plus
Poor DNA recovery may be caused by one or more of the following:
- Incomplete lysis of cells or tissue. Ensure that the appropriate amount of Buffer SKP was used for the amount of cells or tissue. Optionally, the lysate could be heated at 55°C for 10 minutes to assist in lysis.
- Column has become clogged. Do not exceed the recommended amounts of starting materials. The amount of starting material may need to be decreased if the column shows clogging below the recommended levels. See FAQ related to “Clogged Column” below.
- An alternative elution solution was used. It is recommended that the RNA Elution Buffer supplied with this kit be used for maximum RNA recovery.
- Ethanol was not added to the lysate. Ensure that the appropriate amount of ethanol is added to the lysate before binding to the column.
- Ethanol was not added to the Wash Solution A. Ensure that 90 mL of 96 – 100 % ethanol is added to the supplied Wash Solution A prior to use.
- Low RNA content in cells or tissues used. Different tissues and cells have different RNA contents, and thus the expected yield of RNA will vary greatly from these different sources. Please check literature to determine the expected RNA content of your starting material.
- Cell Culture: Cell monolayer was not washed with PBS. Ensure that the cell monolayer is washed with the appropriate amount of PBS in order to remove residual media from cells.
- Yeast: Lyticase was not added to the Resuspension Buffer. Ensure that the appropriate amount of lyticase is added when making the Resuspension Buffer.
- Bacteria and Yeast: All traces of media not removed. Ensure that all media is removed prior to the addition of the Buffer SKP through aspiration.
Column clogging may occur due to:
- Insufficient solubilization of cells or tissues. Ensure that the appropriate amount of lysis buffer was used for the amount of cells or tissue.
- Maximum number of cells or amount of tissue exceeds kit specifications. Refer to specifications to determine if the amount of starting material falls within kit specifications.
- Centrifuge temperature is too low. Ensure that the centrifuge remains at room temperature throughout the procedure. Temperatures below 20°C may cause precipitates to form that can cause the columns to clog.
RNA may be degraded due to:
- RNase contamination. RNases may be introduced during the use of the kit. Ensure proper procedures are followed when working with RNA. Please refer to “Working with RNA” at the beginning of this user guide.
- Procedure not performed quickly enough. In order to maintain the integrity of the RNA, it is important that the procedure be performed quickly. This is especially important for the Cell Lysate Preparation Step in the Animal Tissue protocol, since the RNA in animal tissues is not protected after harvesting until it is disrupted and homogenized.
- Improper storage of the purified RNA. For short-term storage, RNA samples may be stored at –20°C for a few days. It is recommended that samples be stored at –70°C for longer-term storage.
- Tissue samples were frozen improperly. Samples should be flash-frozen in liquid nitrogen and transferred immediately to a -70°C freezer for long-term storage.
- Frozen tissues or cell pellets were allowed to thaw prior to RNA isolation. Do not allow frozen tissues to thaw prior to grinding with the mortar and pestle in order to ensure that the integrity of the RNA is not compromised.
- Lysozyme or lyticase used may not be RNase-free. Ensure that the lysozyme and lyticase being used with this kit are RNase-free, in order to prevent possible problems with RNA degradation.
If the RNA does not perform well in downstream applications, it may be due to one or more of the following:
- RNA was not washed twice with the provided Wash Solution A. Traces of salt from the binding step may remain in the sample if the column is not washed twice with Wash Solution A. Salt may interfere with downstream applications, and thus must be washed from the column.
- Ethanol carryover. Ensure that the dry spin under the RNA Wash procedure is performed, in order to remove traces of ethanol prior to elution. Ethanol is known to interfere with many downstream applications.
Poor protein recovery may be caused by one or more of the following:
- Incorrect pH adjustment of sample. Ensure that the pH of the starting protein sample is adjusted to pH 3.5 or lower after the Binding Buffer A has been added and prior to binding to the column. If necessary, add additional Binding Buffer A.
- Low protein content in the starting materials. Run a 20 µL fraction from the flowthrough (after RNA binding) on an SDS-PAGE gel to estimate the amount of protein present in the sample. In addition, use the entire flowthrough in the protein purification procedure.
Protein may be degraded due to:
- Eluted protein solution was not neutralized. Add 9.3 µL of Protein Neutralizer to each 100 µL of eluted protein to adjust the pH to neutral. Some proteins are sensitive to high pH, such as the elution buffer at pH 12.5.
- Eluted protein was not neutralized quickly enough. If eluted proteins are not used immediately, degradation will occur. We strongly suggest adding Protein Neutralizer to lower the pH promptly.
- Eluted protein was not stored properly. Purified protein needs to be stored at -70°C. Adding protease inhibitors will help enhance protein stability.
Yes, samples preserved in RNAlater can be processed using our RNA/Protein Purification Plus Kit. Norgen Biotek also offers an alternative to RNAlater called RNA preserve (Product number 17260)
Yes, the eluted protein and elution buffer C (+ protein neutralizer) are compatible with applications like Mass Spectrometry.
Citations
| Title | Defining the relationship between cellular and extracellular vesicle (EV) content in breast cancer via an integrative multi-omic analysis |
| Citation | Proteomics 2024. |
| Authors | Rebecca E. Lane1 Darren Korbie1 Kum Kum Khanna2 Ahmed Mohamed2 Michelle M. Hill2 Matt Trau1,3 |
| Title | Effect of Lifelong Exposure to Dietary Plant and Marine Sources of n-3 Polyunsaturated Fatty Acids on Morphologic and Gene Expression Biomarkers of Intestinal Health in Early Life |
| Citation | Nutrients 2024. |
| Authors | Julianna E. Acosta 1,Jessie L. Burns 2,Lyn M. Hillyer 1,Kelsey Van 1,Elaina B. K. Brendel 1,Camille Law 1,David W. L. Ma 1ORCID andJennifer M. Monk 1,* |
| Title | Nintedanib downregulates the profibrotic M2 phenotype in cultured monocyte-derived macrophages obtained from systemic sclerosis patients affected by interstitial lung disease |
| Citation | Arthritis Research & Therapy 2024. |
| Authors | Stefano Soldano, Vanessa Smith, Paola Montagna, Emanuele Gotelli, Rosanna Campitiello, Carmen Pizzorni, Sabrina Paolino, Alberto Sulli, Andrea Cere & Maurizio Cutolo |
| Title | Relative uptake of tomato carotenoids by in vitro intestinal and prostate cancer cells |
| Citation | The Journal of Nutrition 2024. |
| Authors | Moran Ne, Alexander B., Garg S. Marchant N., Hason Na |
| Title | Poly (ADP-Ribose) Polymerase-1 (PARP-1) Induction by Cocaine Is Post- Transcriptionally Regulated by miR-125b |
| Citation | Disorders of the Nervous System 2017. |
| Authors | Dash, S., Balasubramaniam, M., Rana, T., Godino, A., Peck, E. G., Goodwin, J. S., ... & Pandhare, J. (2017). |
| Title | Quantification of spatiotemporal patterns of Ras isoform expression during development |
| Citation | Scientific Reports 2017. |
| Authors | Newlaczyl, A. U., Coulson, J. M., & Prior, I. A. |
| Title | Expression of Tachykinins and Tachykinin Receptors and Interaction with Kisspeptin in Human Granulosa and Cumulus Cells |
| Citation | Biology of Reproduction 2016. |
| Authors | Garcia-Ortega, J., Pinto, F. M., Prados, N., Bello, A. R., Almeida, T. A., Fernández-Sánchez, M., & Candenas, L |
| Title | Wingless (WNT) signaling is a progesterone target for rat uterine stromal cell proliferation |
| Citation | Journal of Endocrinology 2016. |
| Authors | Rider, V., Talbott, A., Bhusri, A., Krumsich, Z., Foster, S., Wormington, J., & Kimler, B |
| Title | Estrogen induced changes in uterine brain-derived neurotrophic factor and its receptors |
| Citation | Human Reproduction 2015. |
| Authors | JM Wessels, NA Leyland, SK Agarwal, WG Foster |
| Title | The Brain-Uterus Connection: Brain Derived Neurotrophic Factor (BDNF) and Its Receptor (Ntrk2) Are Conserved in the Mammalian Uterus |
| Citation | PLoS One 2014. |
| Authors | JM Wessels, L Wu, NA Leyland, H Wang, WG Foster |