Revisiting the Gold Standard for Stool Sample Storage
With the advent of microbiome studies, stool sample collection and preservation have been highlighted as key steps in ensuring microbiome authenticity. Freezing at -80°C has been widely accepted as the gold standard for stool sample storage in a variety of research and clinical applications. However, this method of storage renders host cells and microorganisms susceptible to deleterious effects such as freeze-shock, prolonged freezing, and freeze-thaw. The combination of these freezing and thaw effects leads to cell death, a selection for freeze-tolerant microbes, and concomitant introduction of bias in microbiome studies (Walker et al., 2006). Therein, further investigation into the gold standard method for stool sample storage is required.
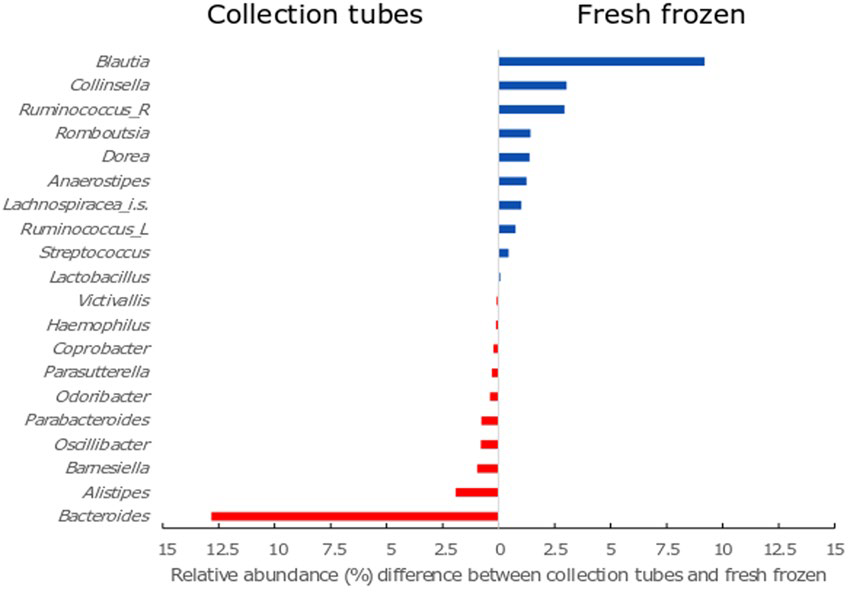
Watson et al. (2019) explored the issue of stool sample storage in their study comparing microbiome profiles of stool preserved with Norgen’s Stool Nucleic Acid Preservative to frozen stool. Samples were collected by the donors and immediately stored in preservative or at -20ºC. Preserved samples were shipped at ambient temperature, while frozen samples were shipped in transportable freezers. The 16S rRNA gene was subsequently sequenced from preserved and frozen samples. The authors found a greater proportion of Gram-positive bacteria in frozen samples, while the Norgen-preserved samples exhibited the opposite pattern. As previously noted, freezing selects for freeze-tolerant microbes and leads to the cellular lysis of freeze-susceptible microbes. Exposed DNA and RNA are then subject to free nucleases, which can lead to differences in microbial profiles from the same donor and thereby threaten study validity.
NORBLOG
Want to hear more from Norgen?
Join over 10,000 scientists, bioinformaticians, and researchers who receive our exclusive deals, industry updates, and more, directly to their inbox.
For a limited time, subscribe and SAVE 10% on your next purchase!
SIGN UP
Figure 1. Twenty bacterial taxa that exhibited a statistically significant (P < 0.050) difference between % relative abundance in the frozen collection method compared to the tube collection method. The top ten genera (blue) are all Gram-positive and at a relative higher abundance in the fresh frozen samples. The bottom ten genera (red) are all Gram-negative and at a relative higher abundance in the collection tube samples. Ruminococcus_R – Ruminococcus with the Ruminococcaceae family; Ruminococcus_L – Ruminococcus with the Lachnospiraceae family; Lachnospiraceae_i.s.- Lachnospiraceae incertae sedis. (Watson et al., 2019).
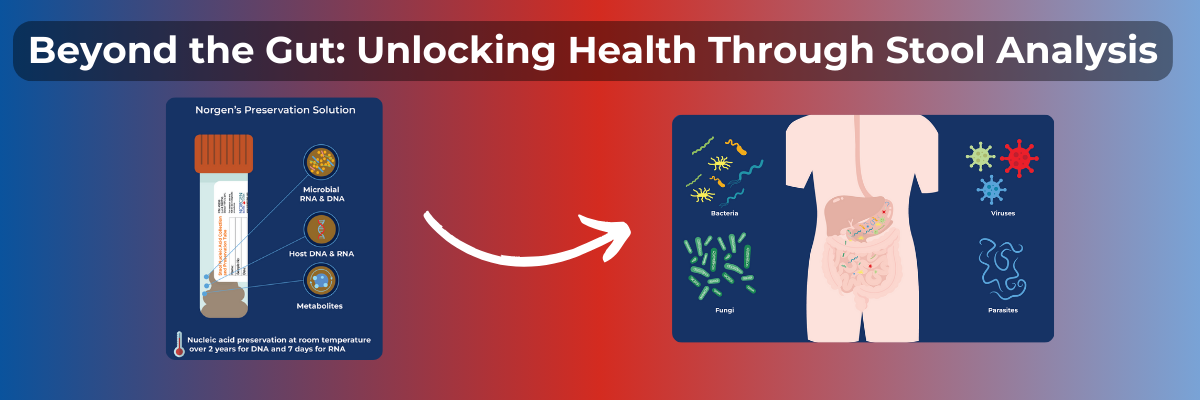
Comparisons of several preservatives—including Norgen’s Stool Nucleic Acid Preservative—to frozen fecal samples has led to the recommendation of the use of a preservative such as Norgen’s Stool Nucleic Acid Preservative for optimal microbiome authenticity (Chen et al., 2019). When processing of fresh stool is not possible, preservation with Norgen’s Stool Nucleic Acid Collection and Preservation Tubes offers nucleic acid preservation at room temperature and protection from freeze-thaw effects. With a larger sample capacity and minimal sample dilution compared to other options, feel confident in collecting representative samples for your microbiome studies.
-
Chen, Z., Hui, P. C., Hui, M., Yeoh, Y. K., Wong, P. Y., Chan, M. C. W., Wong, M. C. S., Ng, S. C., Chan, F. K. L., & Chan, P. K. S. (2019). Impact of Preservation Method and 16S rRNA Hypervariable Region on Gut Microbiota Profiling. MSystems, 4(1), e00271-18.
-
Walker, V. K., Palmer, G. R., & Voordouw, G. (2006). Freeze-Thaw Tolerance and Clues to the Winter Survival of a Soil Community. Applied and Environmental Microbiology, 72(3), 1784–1792.
-
Watson, E.-J., Giles, J., Scherer, B. L., & Blatchford, P. (2019). Human faecal collection methods demonstrate a bias in microbiome composition by cell wall structure. Scientific Reports, 9(1), 1–8.